Dibutyryl-cAMP Alters the Expression of Signalling Molecules, Leading to a Metaphase-I Arrest in Rat Oocytes Cultured In Vitro
Alka Sharma1, Pawan K Dubey2 and Anima Tripathi1*
1Zoology Section, MMV, Department of Zoology, Banaras Hindu University, Varanasi- 221005, U.P., India
2Centre for Genetic Disorders, Institute of Science, Banaras Hindu University, Uttar Pradesh, India
Submission: May 30, 2023; Published:July 26, 2023
*Corresponding author: Anima Tripathi, Zoology Section, MMV, Department of Zoology, Banaras Hindu University, Varanasi- 221005, U.P, India, Email: animatripathi@bhu.ac.in
How to cite this article: Alka Sharma, Pawan K Dubey Anima Tripathi. Dibutyryl-cAMP Alters the Expression of Signalling Molecules, Leading to a Metaphase-I Arrest in Rat Oocytes Cultured In Vitro. and Task Shifting. Glob J Reprod Med. 2023; 10(3):8055779. DOI: 10.19080/GJORM.2023.10.555787.
Abstract
One of the most important intracellular signalling molecules, cyclic adenosine monophosphate (cAMP), acts as a second messenger in the activation of gonadotrophins. In the meiotic cell cycle, the function of the cAMP analogue dibutyryl cAMP (db-cAMP) is not well understood beyond the metaphase-I (M-I) stage. In this section of the investigation, we test whether db-cAMP may induce a transient arrest of meiosis at the M-I stage. In cultured rat oocytes, we modify kinases, signal molecules, and cell cycle regulators. We harvested M-I-arrested cumulus oocyte complexes (COCs) from rats’ ovaries after superovulation. These oocytes were grown in fresh complete medium with db-cAMP at 0.125, 0.25, 0.5, and 1mM. Oocytes were treated with db-cAMP, and their morphology, meiotic phases, phosphorylation of cyclin-dependent kinase 1 (Cdk1) and cyclinB1, levels of cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), intracellular reactive oxygen species (ROS), Calcium Ca2+, mitochondrial membrane potential, and apoptotic status were all examined. In our in vitro investigation, 1 mM db-cAMP dramatically lowered Thr-14/Tyr-15 pCdk1, causing meiotic arrest in the M-I stage with competent oocytes for up to 12 hours. when db-cAMP treatment, intra-oocyte cyclic nucleotides increased, causing meiotic arrest. Control oocytes resumed meiosis when ROS and Ca2+ increased. Due of damaged mitochondria, long-term db-cAMP treatment causes apoptosis in oocytes. We also study db-cAMP concentrations and periods that decrease spontaneous meiosis resumption after M-I arrest. This study introduces an in vitro approach for meiotic arrest with equally dispersed mitochondria during M-I stage. This may increase oocyte competency in ART practices.
Keywords: Metaphase-I arrest; db-cAMP; Signal molecules; Oocyte quality; Mitochondrial membrane potential
Abbreviations: COCS: Cumulus Oocyte Complexes; ROS: Reactive Oxygen Species; cAMP: Cyclic Adenosine Monophosphate; cGMP: Cyclic Guanosine Monophosphate; MAPK: Mitogen-Activated Protein Kinase; MPF: Maturation-Promoting Factor
Introduction
Among the many exciting aspects and qualities that make the rat an interesting model for investigating meiotic cell cycle control is the fact that, in in vitro culture environments, the oocyte does not go beyond the metaphase-I stage (M-I) [1,2]. In mammals, the voyage from metaphase-I (M-I) to metaphase-II (M-II) is crucial because the egg extrudes the first polar body (PB-I) and becomes haploid gamete. PB-I extrusion and meiotic cell cycle progression from M-I to M-II are poorly understood. Therefore, it is critical for both fundamental research and ART to understand how mammalian oocytes undergo meiotic maturation. Meiotic arrest and resumption occur in oocytes via a series of chemical cascades that are regulated by a variety of signalling molecules produced by follicular cells [3,4]. The gonadotropins produced by the pituitary gland also play an important role in the cascade of events that ends in meiotic resumption. Several protein kinases (enzymes) play a crucial role in keeping mammalian oocytes in a state of meiotic arrest. Key protein kinases include protein kinase A (PKA), protein kinase B (PKB), protein kinase C (PKC), and mitogen-activated protein kinase (MAPK) in sustaining meiotic cell cycle progression in mammalian oocytes [5].
To communicate with oocytes, adenylate cyclase uses gonadotrophins to produce the second messenger 3’,5’-cyclic adenosine monophosphate (cAMP) [6-8]. Rat granulosa cells and oocytes contain a differentiation factor termed cAMP, which governs the progression of the meiotic cell cycle by maintaining the meiotic arrest [3,9,10]. During the diplotene and M-I phases, meiosis is arrested, but when cAMP and 3′,5′-cyclic guanosine monophosphate (cGMP) concentrations drop, meiosis resumes [11]. Reactive oxygen species (ROS) generation is linked to decreased levels of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) following spontaneous meiotic restart after meiotic arrest in oocytes cultured in vitro [12]. It’s well knowledge that reactive oxygen species (ROS) function as signalling molecules in many different types of cells, including mammalian germ cells. Mild increases in ROS aid in the progression of the meiotic cell cycle during in vitro culture [13].
An increase in cAMP levels activates cAMP-dependent protein kinase A, which in turn activates the maturation-promoting factor (MPF) [6]. Oocytes are unable to enter the M-I arrest phase of meiosis due to this. MPF regulates oocyte meiosis in all mammals [14,15]. CDK1, a component of the MPF, is essential for meiotic resumption [16]. Meiotic resumption in M-I arrested oocytes occurs when the MPF complex is functional. Stabilisation of MPF requires cyclin B1 contact with Cdk1, phosphorylation at the Thr161 residue, and dephosphorylation at the Thr-14/Tyr-15 of Cdk1 [17]. Dephosphorylation of Thr161 and phosphorylation of Cdk1’s Thr-14/Tyr-15 residues turn MPF unstable, and its breakdown through ubiquitin-mediated proteolysis triggers exit from metaphase-I (EM-I) [18,19].
Meiotic competence allows an egg to develop into a viable organism, thus it’s crucial. Meiotically competent oocytes have the potential to improve ART success rates in most mammalian species [20]. Oocytes may restart meiosis on their own [21] if they are separated from their follicular environment. Findings suggest that FSH improves oocyte development via a cAMP-mediated pathway while initially delaying nuclear maturation [22-24]. Dibutyryl cAMP sodium salt (db-cAMP), a membrane-permeable cAMP analogue, and 3-isobutyl-1-methylxanthine (IBMX), a type 3 phosphodiesterase inhibitor, have both been used in in vitro maturation (IVM) to increase cAMP levels, delay meiosis, and subsequently regulate nuclear and cytoplasmic oocyte development [6, 25, 26].
We hypothesise that in vitro cultured oocytes would be less capable of maturing if their cAMP levels were suddenly lowered. Oocyte morphology, mitochondrial membrane potential, and early apoptosis were also analysed as a function of db-cAMP delivery during in vitro growth. Oocytes in the M-I stage were essentially stopped at the arbitrary exit for up to 12 hours, while still being alive and having equally dispersed mitochondria. There is a lack of information on the method and mechanism of action in M-I rat oocytes. This study examined the impact of db-cAMP on spontaneous meiotic restart from the M-I stage in rat oocytes during in vitro culture at different time hours to better understand the mechanism behind db-cAMP treatment on oocyte maturation at different culture durations.
Chemicals and culture media
Chemicals and culture media
We purchased antibiotics and culture media from HiMedia. With the exception of the compounds specifically named, all others were acquired from the Sigma Chemical Co. located in St. Louis, Missouri. The pH of the M-199 culture media was adjusted to 7.2 ± 0.05 by putting in sodium bicarbonate (0.035% w/v) per the manual instructions. After that, we added glutamate, penicillin, and streptomycin (Cat. no. A007, HiMedia) to the culture mix to inhibit further microbial growth.
Dibutyryl Cyclic AMP (db-cAMP) working concentration preparation
Dimethyl sulfoxide (DMSO, 0.1%) was used to dissolve the sodium salt of db-cAMP in order to generate a 1 mM stock solution. The stock solution was diluted using the media to attain the working concentrations (0, 0.125, 0.25, 0.5, and 1 mM) for in vitro investigations. The working concentrations were prepared for 5 minutes at 37°C before use.
Animals, oocytes Collection and Culture
Charles-Foster strain female rats of sexually immature age (22- 24 days old; 45 ± 5 g body weight) were housed and maintained with ad libitum access to food and water per conventional husbandry practises. Subcutaneous injections of 20 IU of pregnant mare’s serum gonadotropin (PMSG) were given to rats for 48 hours, and then 20 IU of human chorionic gonadotrophin (hCG) were given to them 10 hours later (Superovulation protocol). The ovaries were removed from the rats that were killed through cervical dislocation and placed in petri plates with preheated media.
Under a stereomicroscope (SMZ800N, Nikon, Tokyo, Japan), the ovary was perforated with a 26-gauge needle connected to a 1 mL tuberculin syringe, and the ovary and fallopian tube were removed and deposited in the pre-warmed M-199 medium in a 35 mm Petri dish. Oocytes in the M-I arrest state were characterised morphologically due to the absence of GV and nucleus in the cytoplasm. Microtubing was linked to disposable glass micropipettes, which were used to collect the M-I arrested oocytes. After a brief incubation at 37°C, they were transferred to fresh culture medium containing 0.01% hyaluronidase. Oocytes that had been M-I arrested were used for in vitro tests after being washed three times.
Evaluation of oocyte meiotic status
With the use of Hoechst-33342 staining, we were able to assess an oocyte’s meiotic state, as verified by its chromosomal status. After two washes in phosphate-buffered saline (PBS), 6-8 oocytes were incubated with 10 μg/ml Hoechst 33342 for 5 minutes at 37 °C. For chromosomal analysis, we used a fluorescence microscope (Eclipse Ni, Nikon, Tokyo, Japan) set to 350 nm, and for oocyte morphology, we used a phase contrast microscope (Eclipse E200, Nikon, Tokyo, Japan). Meiotic stage was verified by three independent tests.
The effect of db-cAMP on spontaneous meiotic resumption from M-I arrested oocytes
A group of (10-14) oocytes were placed in petri dishes with various concentrations of db-cAMP (0, 0.125, 0.25, 0.5, and 1 mM), with DMSO serving as the control. The petri dishes followed by placing in a CO2 incubator (Galaxy 170R, Eppendorf, Hamburg, Germany) set at 37 °C and were placed for incubation for up to 12-24 hours. After incubation for varying amounts of time, morphological changes in the oocytes were analysed with a phase contrast microscope. At least three sets of experiments were performed to ensure the accuracy of the results.
Immunocytochemistry for cAMP analysis
The concentration of cAMP was evaluated by employing a monoclonal antibody. For this objective, oocytes (n=5-6) from the control and db-cAMP treatment groups are fixed with 4% buffered paraformaldehyde and then air-dried. Following a threestep PBS washing procedure, slides were treated with 100 μl of Triton X-100 (0.01% in PBS) at room temperature for 5 minutes. After being washed twice or thrice with PBS, the slides were then treated with sodium citrate solution (0.01 M) at 37°C for 10 mints. After a second PBS wash, the slides were incubated with a blocking buffer (2.5% PBS-BSA solution) at 37°C for 30 minutes. Next, slides were treated with 100 μl of diluted (1:500 dilution in blocking solution) primary antibody (cAMP, mouse monoclonal, sc-73761) after a PBS wash. The intensity of fluorescence was measured at 465 nm using a fluorescence microscope. Oocytes were subjected to corrected total cell fluorescence (CTCF) analysis to verify the findings, and at least three independent sets of tests were conducted. Simply said, cell-to-background integrated fluorescence (CTCF) is the cell’s total fluorescence.
Evaluation of MPF evel
Our method for analysing the phosphorylation of Cdk1 and cyclin B1 levels in db-cAMP-treated oocytes was described in [17], and it included the use of extremely specific antibodies from Santa Cruz Biotechnology (Dallas, TX, USA). Thr-14/Tyr- 15 p-Cdc2p34 rabbit polyclonal antibody (sc-12340) and Thr- 161 p-Cdc2p34 rabbit polyclonal antibody (sc-12341) were both generated against sequences containing Thr-14 and Tyr-15, respectively. p-34Cdc2p34 (PSTAIRE) rabbit polyclonal antibody (sc-53) was generated against the conserved PSTAIRE domain of Cdc2. Following incubation, slides were rinsed thrice with PBS, and then subjected to 100μl of specific anti-rabbit fluorescein isothiocyanate (FITC)-labelled (sc-3839) secondary antibody for detecting Thr-14/Tyr-15, Thr-161, as well as total phosphorylated Cdk1 and cyclin B1 levels, and anti-mouse TRITC-labelled (sc- 3796) secondary antibody for detection of β-actin at 37°C for 1 hour (1:1000 dilutions in blocking buffer). After incubating the slides for an hr, the fluorescence intensity was evaluated using a fluorescent microscope at 465 nm (FITC) and 540 nm (TRITC) wavelengths, respectively. The slides were washed three times in PBS. To correlate the data, we show you example photos from each of the three independent runs of the experiment, where 4-5 denuded oocytes were subjected to CTCF analysis using Image J software (version 1.44 from National Institutes of Health, Bethesda, USA).
Quantitative assessment of cAMP and cGMP Levels
Total arrest in the M-I stage was seen in the treatment group for as long as 12 hours, but in the control group, cyclic nucleotide levels were only measured in the control and 1 mM db-cAMPtreated groups. Eight to ten oocytes were taken from each group and deposited in lysis buffer (20 mM Tris-HCL, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 1% Triton-X 100; pH = 7.2) for quantitative analysis. All the samples, standards, and reagents were prepared following the procedures outlined in the relevant business handbook. The results section displays the cAMP and cGMP concentrations in terms of pmol/mg protein. R&D Systems Inc. ELISA kit for cAMP and cGMP analysis (Cat. No. KGE002B for cAMP and KGE003 for cGMP, respectively) from the United States.
Measurement of total ROS level
Following our previously published technique [17, 27], the total ROS level was assessed using 2’, 7’-dichlorodihydrofluorescein diacetate (H2DCFDA). For this, 15 min at 37oC in a CO2 incubator were spent exposing 10-12 oocytes from each control and treated group (12 and 24 hours) of 1 mM db-cAMP to H2DCFDA (10 μM). Oocytes were rinsed thrice with PBS before being subjected to a fluorescence microscope analysis of DCF fluorescence at 485 nm excitation and 520 nm emissions. Three independent experiments were performed in order to verify the findings, and CTCF analysis was carried out using ImageJ software (version 1.44; National Institutes of Health, Bethesda, USA).
Fluo-3 AM-Based Intracellular Ca2+ Analysis.
Following a methodology that had already been described [20], the intracellular Ca2+ level was examined in the control and treatment groups. In a nutshell, culture media containing 50 μM Fluo-3 AM were exposed to 10-12 oocytes from the control and 1 mM db–cAMP treated groups for 12 and 24 hours, respectively, at 37 °C in a CO2 incubator. Oocytes were then taken out and thoroughly cleaned with PBS three times before Fluo-3 fluorescence was measured at 488 nm excitation and 520 nm emission under a fluorescent microscope (Nikon, Eclipse; E-80i, Japan). Using the Image J Software (version 1.44 from the National Institutes of Health, Bethesda, USA), the CTCF of oocytes from three different experiments were determined.
Monitoring of mitochondrial membrane potential (ΔΨ) of db-cAMP treated oocytes
The viability of the cell was further evaluated for up to 24 hours with 1 mM of db-cAMP among all the doses. Oocytes were stained with JC-1, a reporter dye for the inner mitochondrial membrane potential, as directed by the manufacturer. In brief, treated oocytes were incubated with JC-1 for 20 minutes after being produced to a final concentration of 1 M in dilution buffer. An inverted fluorescence microscope (EVOS FL, Life Technologies) was used to observe the stained oocytes. By measuring the total fluorescence of the whole oocyte, fluorescence analysis was conducted. The CTCF approach was used to normalise the mean value for each fluorescence to the measuring area [28]. Individual oocyte values from three treatments were expressed as total red CTCF [29].
Staining With Acridine Orange and Propidium Iodide to Identify Apoptotic Cells.
Acridine orange (AO) and propidium iodide (PI) staining confirmed apoptotic profiles as a consequence of morphological alterations in the db-cAMP-treated oocytes at different time points. Briefly, oocytes from each group that had been treated with dbcAMP were washed in PBS and then stained for 10 minutes with an AO/PI mix (1 μg/ml in PBS). Fluorescence was observed using an inverted fluorescence microscope (EVOS FL, Life Technologies) in three separate investigations [28].
Statistical analysis
The data were collected from three separate studies, and the results were reported as the mean ± standard error of mean (S.E.M) Before doing statistical analysis, all percentage data were transformed using the arcsine square root. The chi-square (χ2) testing was used to compare the rates of meiotic resumption between the control and db-cAMP-treated groups (Table 1). SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, U.S.A.), was used to perform a one-way ANOVA (P 0.05), followed by the Bonferroni test on a subset of the data, and the student’s t-test on the remaining data. A substantial change from the control group or the treatment group is indicated by a “*” or “***,” respectively. P < 0.05 and P < 0.001were used as criteria for statistical significance.
Results
Oocytes undergo morphological changes during in vitro maturation
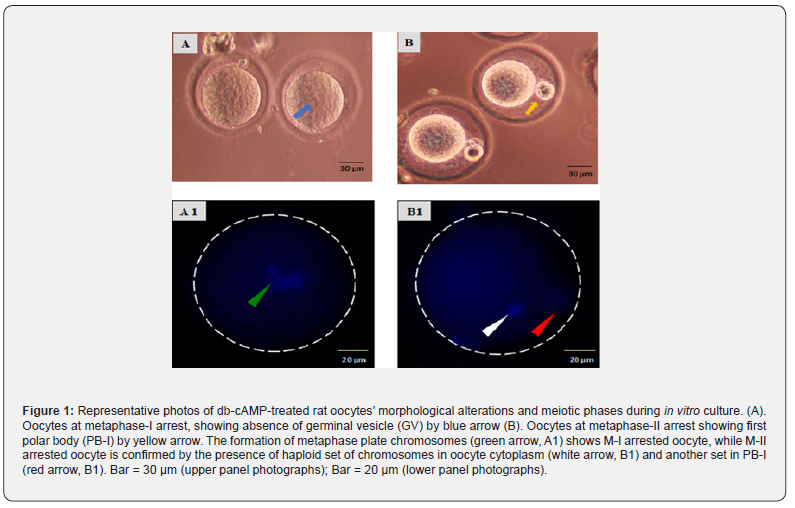
As shown in (Figure 1), oocytes collected after treatment with 20 IU PMSG and 20 IU hCG exhibited M-I arrest, as shown by the lack of GV (blue arrow; Figure 1A). Oocytes in the control group revealed the disappearance of GV and the existence of PB-I to demonstrate M-II arrest (yellow arrow; Figure 1B) after 5-6 hours of in vitro culture. Hoechst-33342 staining further validated their meiotic status, which included phases such as M-I arrest (dbcAMP treated group) (Figure 1, A1) and M-II arrest (Figure1, B1).
During in vitro Culture, db-cAMP inhibited spontaneous meiotic resumption in a concentration-dependent manner
As shown in (Figure 2), The germinal vesicle (red arrow, Figure 2A) is absent in M-I oocytes. Oocytes in the control group spontaneously exited M-I arrest and had PB-I (76.32 ± 5.802%; blue arrow; Figure 2B) after 12 hours of in vitro culture. Maximum inhibition of meiotic resumption was noticed in 1 mM concentration of db-cAMP (1 mM) up to 12 hours, with proper oocyte morphology observed till 18 hours (5.31 ± 0.82%), and demonstrating oocytes at complete M-I arrest evidenced by the absence of germinal vesicle (black arrow; Figure 2F), whereas in 0.5 mM, 0.25 mM, and 0.125mM concentration of db-cAMP shows spontaneous meiotic resumption from M-I arrest evidenced by exit from M-I arrest (EM-I) (green arrow; Figure 2E) and presence of PB-I (yellow arrow; Figure 2D and 2C). The addition of db-cAMP during in vitro culture of M-I arrested oocytes at 12 hours significantly reduced spontaneous meiotic restart (One way ANOVA, F = 51.278, p < 0.001; Figure 2G) in a concentrationdependent manner.
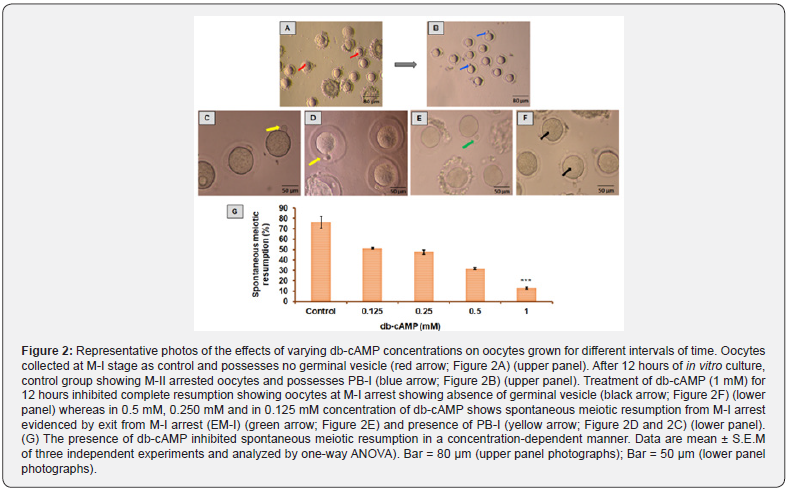
The results represent the mean standard error of the mean across three investigations and were analysed using a one-way ANOVA. Bar = 80 m (images in top panel); Bar = 50 m (pictures in bottom panel). Oocyte resumption after in vitro culture was 77.11% in the control group and 7.69% in the db-cAMP (1 mM) treated group. In contrast, the 1 mM db-cAMP treated group showed statistical significance when compared to the control group using the χ2 test (χ2 calculated 4.33 > χ2 critical 3.84; p < 0.05). Therefore, there is a notable difference between the 1 mM of db-cAMP and the control.
Camp immunofluorescence intensity increased in dbcAMP- treated oocytes
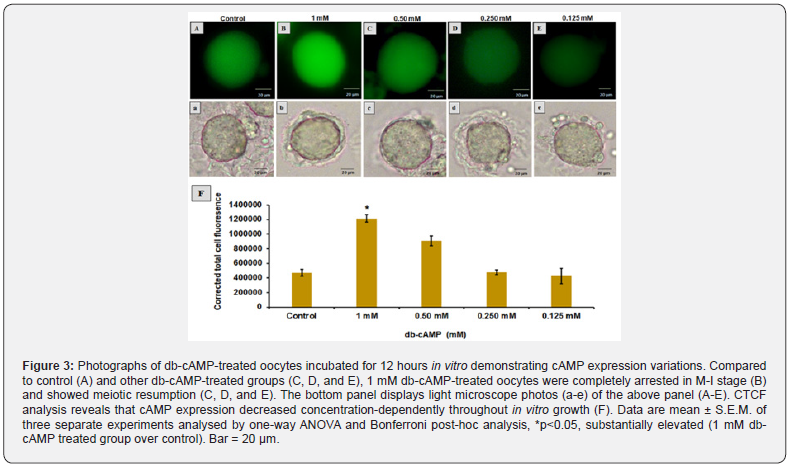
As shown in (Figure 3), Compared to the control (Figure 3A) and another db-cAMP treated group in which cAMP expression decreased as the concentration of db-cAMP decreased (Figure 3CE), and where meiotic resumption was also observed, the 1 mM db-cAMP treated group showed a significant (p < 0.05) increase in cAMP expression (one-way ANOVA, F = 32.15; p < 0.05). The images from the light microscope (Figure 3a-e) in the top panel are shown in the bottom panel. Figure 3F shows that the CTCF analysis confirms these results in more depth.
In The db-cAMP-treated Group, MPF Stabilisation Leads to Meiotic Arrest
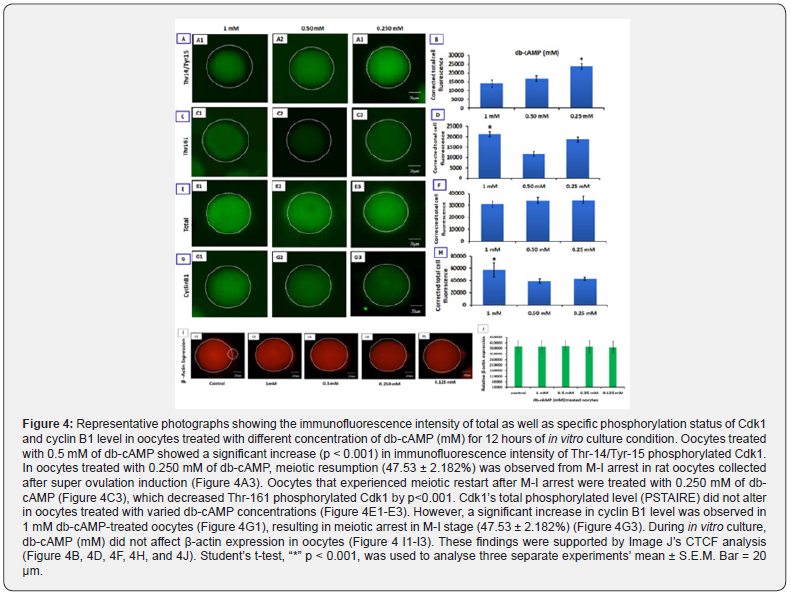
Oocytes treated with 0.5 mM db-cAMP exhibited a considerable rise (p<0.001) in the immunofluorescence intensity of Thr-14/ Tyr-15 phosphorylated Cdk1 level, but oocytes treated with 1 mM db-cAMP remained entirely in M-I arrest until 12 hours of in vitro growth (Figure 4A1; A2). Oocytes treated with 1 mM of db-cAMP for 5 hours show a decrease in Thr-14/Tyr-15 phosphorylated Cdk1 level, while oocytes treated with different concentrations of db-cAMP for 12 hours maintained MPF stabilisation, resulting in meiotic arrest at the M-I stage (Figure 4 C1-C3). Oocytes treated with varying amounts of db-cAMP for 12 hours showed no difference in the total phosphorylated level (PSTAIRE) of Cdk1 (Figure 4 E1-E3). Meiotic arrest during the M-I stage is caused by an increase in cyclin B1 levels, as shown by the dramatic rise in cyclin B1 levels in oocytes treated with 1 mM db-cAMP (Figure 4G1) compared to 0.25 mM db-cAMP treated oocytes (Figure 4G3). Meiotic arrest at the M-I stage is induced by 1 mM db-cAMP in oocytes, and competent oocytes remain in arrest for up to 12 hours of in vitro culture, as shown by CTCF analysis (Figures. 4B, 4D, 4F, and 4H).
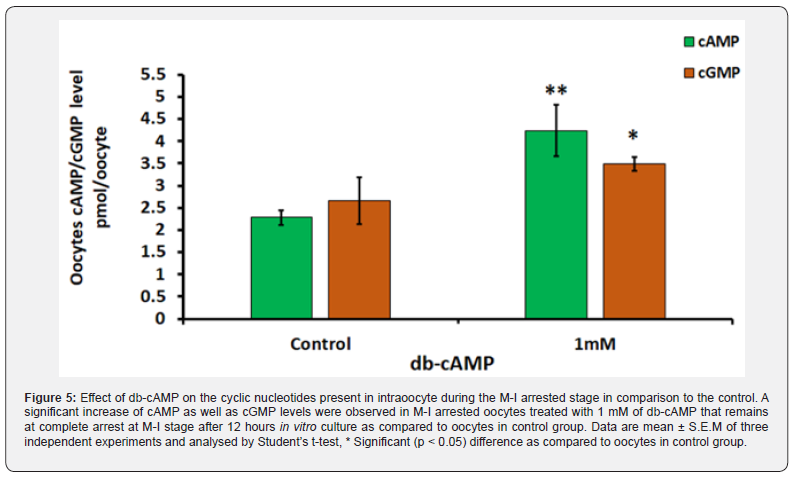
Meiotic Arrest Occurs When Intraoocyte Cyclic Nucleotides Rise
As shown in (Figure 5), oocytes arrested at the M-I stage and treated with 1 mM of db-cAMP show a significant (p < 0.05) of cAMP (4.23 ± 0.57 pmol /oocyte) and cGMP (3.485 ± 0.15 pmol /oocyte) levels when in contrast to the control group of cAMP (2.28 ± 0.17 pmol/oocyte; Figure 5) and cGMP (2.67 ± 0.52 pmol/ oocyte; Figure 5). When M-I arrested oocytes were treated with 1 mM db-cAMP for 12 hours in vitro, no spontaneous meiotic restart was seen in comparison to the control group (Figure 5). Three independent analyses confirmed these results.
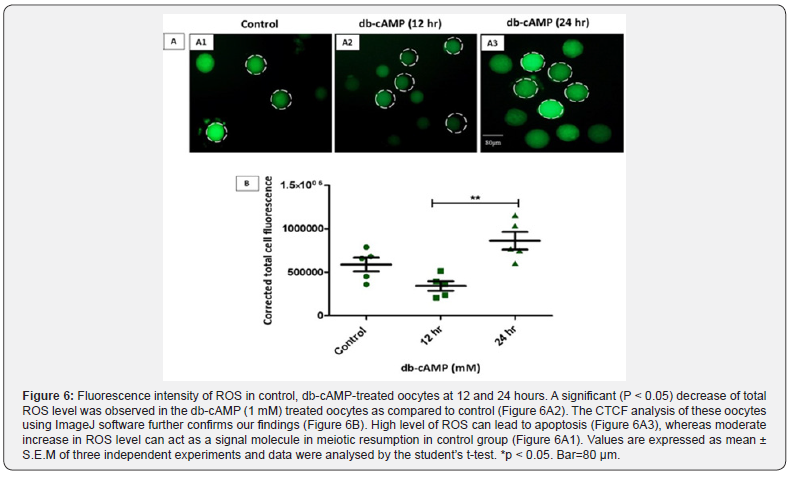
Variations in the levels of total ROS
A comparison of the total ROS level in control, 12-hours, and 24-hours groups of oocytes that were treated with db-cAMP is shown in (Figure 6). This comparison was carried out using the fluorescent dye H2DCFDA. When compared to the control group, the ROS level in the oocytes that had been treated with 1 mM dbcAMP showed a substantial (p < 0.05) drop when examined by the Figure 6A2 diagram. The CTCF analysis conducted on these oocytes using the ImageJ programme has provided support for our previous results (Figure 6B). Apoptosis may be triggered by high quantities of reactive oxygen species (Figure 6A3). However, minor increases in ROS may operate as a signal molecule for the control group’s meiotic restart (Figure 6A1) in the experiment.
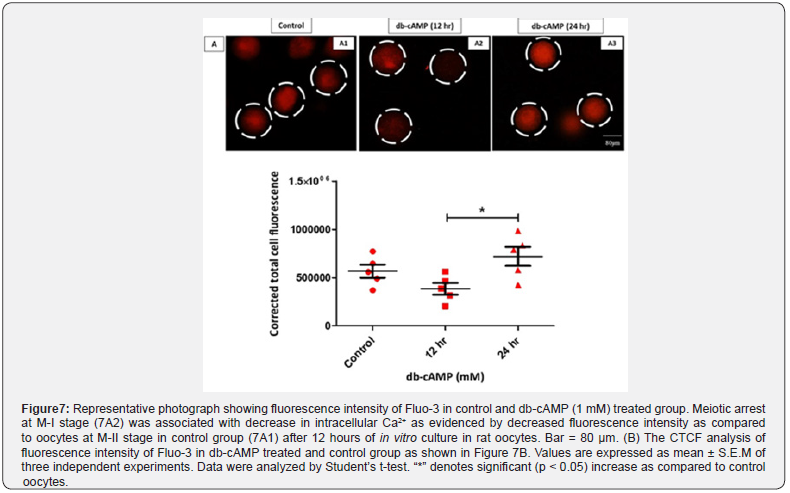
A Change in Intracellular Ca2+ Level
Oocytes treated with 1 mM db-cAMP and grown for up to 12 hours stayed in the M-I arrested stage (Fig. 7A2), as contrasted to the control group (Figure 7A1; M-II stage), as indicated in (Figure 7). This was statistically significant (p < 0.05). After 24 hours of treatment with 1mM db-cAMP, a significantly higher amount of free intracellular Ca2+ was detected inside the deformed cytoplasm condition (1.92 times, Figure. 7A3) as compared to the control group. Our results are supported by the CTCF analysis of three separate trials performed in Image J (Version 1.3) (Figure 7B).
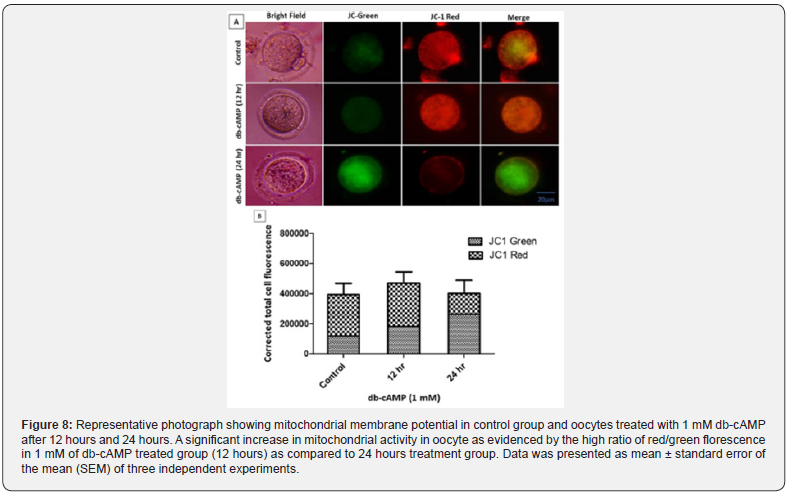
Long-Term db-cAMP Exposure Decreased Oocyte Mitochondrial Activity
Red fluorescence is expressed more strongly by active mitochondria due to the increased accumulation of JC-1 dye in mitochondria, whereas green fluorescence is expressed by less active mitochondria [30]. Figure 8A shows that compared to dbcAMP (1 mM) treated oocytes cultured for 24 hours, db-cAMP (1 mM) treated oocytes treated for up to 12 hours dramatically enhanced mitochondrial activity. However, compared to a 12-hours culture of 1 mM db-cAMP and the control group, a prolonged culture in 1 mM db-cAMP treated oocytes drastically decreased mitochondrial activity (ΔΨ), as seen by a low red/green fluorescence ratio (lower panel).
Acridine Orange/Propidium Iodide Staining Detects Apoptosis in db-cAMP-Treated Oocytes
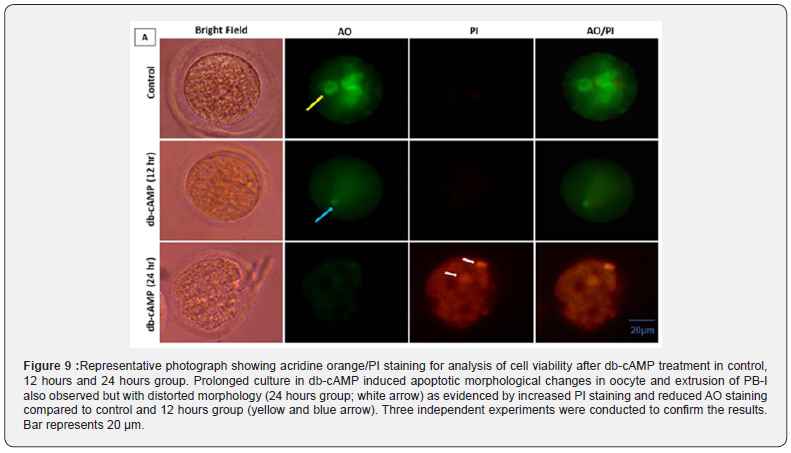
Oocytes treated with 1 mM db-cAMP and grown for 24 hours as opposed to 12 hours in the 1 mM treatment and control groups showed signs of apoptosis. We carried out AO/PI staining to confirm apoptosis due to morphological alterations in respected group. Results showed that the 24 hours culture group of dbcAMP treatment enhanced apoptosis in oocytes as shown by an increase in the intensity of red fluorescence for PI in comparison to control and 12 hours group oocytes (Figure 9; bottom panel). As shown in (Figure 9), the oocytes exposed to 1 mM db-cAMP for 12 hours showed AO green rather than PI red. However, this suggests proper oocyte morphology and integrity for 12-18 hours. According to our research, a 1 mM db-cAMP treatment can prolong the M-I arrest for 12 to 18 hours while maintaining proper oocyte shape, compared to a 24-hours culture, which revealed oocytes with deformed morphology and membrane blebbing [31]. This extended db-cAMP (1 mM) culture of oocytes made them unfit for fertilization and IVF.
Discussion
Crosstalk between several signal molecules is crucial in modifying mammalian oocyte physiology [32]. These signal molecules are produced by the granulosa cells surrounding the oocyte or by the oocyte itself [33,34]. Changes in these signal molecule levels may determine whether an oocyte experiences meiotic arrest or resumption as the meiotic cell cycle progresses [14, 35]. Major signal molecules needed for the meiotic cell cycle progression in mammalian oocytes include cAMP, cGMP, ROS, and Ca2+ [36]. In oocytes, the meiotic cell cycle may be affected by alterations in the signal molecules that regulate the MPF stabilization and destabilization and Cdk1 activity [32, 37].
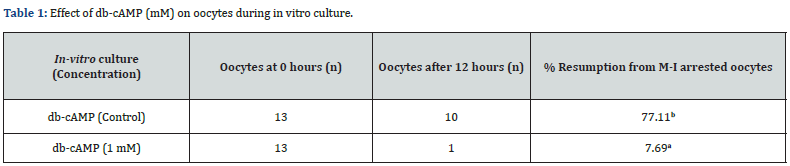
“n” represents the total number of oocytes taken during the in vitro culture. ab, values with different superscript characters last column indicate a statistically significant difference (P < 0.05). Data were analysed using the χ2 test and were obtained by performing 3 replicates. db-cAMP, dibutyryl cAMP; M-I, Metaphase-I.
Alterations in the concentration of a variety of signal molecules may have a direct or indirect effect on MPF stabilisation or destabilisation [37-39]. Our data demonstrate that stabilisation of MPF may be maintained and phosphorylation of Thr-14 and Tyr- 15 in oocytes treated with 1 mM db-cAMP for 12 hours. Oocytes cultured with 1 mM db-cAMP for 12 hours showed a substantial increase in the level of Thr-161 of Cdk1 compared to oocytes cultured with other doses of db-cAMP, resulting in meiotic arrest in the M-I stage. Similarly, oocytes treated with 1 mM db-cAMP for 12 hours showed a significant decrease in cyclin B1 level when compared with 0.25 mM db-cAMP treated oocytes; this indicates the decrease in the level of cyclin B1, which causes a meiotic arrest and keeps M-I arrest in the 1 mM db-cAMP treated group [40, 41]. Oocytes must complete the transition from the M-I to M-II stage of meiosis in order to release PB-I and become the correct gamete [42, 43]. Several physiological variables influence oocyte meiosis as the oocyte undergoes this trip. Ensuring meiotic arrest and meiotic resumption by adjusting the amount of an MPF [40], cAMP is one of the essential physiological components of oocyte meiosis that governs meiotic competence of oocytes. The oocyte protein phosphatase (MPF) activity is regulated by cAMP-dependent protein kinase A (PKA) [37].
cAMP in oocytes controls meiotic arrest [9]. Several investigations show that high intraoocyte cAMP levels maintain meiotic arrest in mature oocytes, whereas low levels resume meiosis [41]. Increasing the dose of db-cAMP kept the oocyte in total arrest for up to 12 hours at 1 mM (5.31 ± 0.82%) in multiple in vitro tests. Compared to 1 mM db-cAMP-treated oocytes, control oocytes display meiotic resumption (76.32 ± 5.802%) and PB-I at the M-II stage. These groups (0.5, 0.25, and 0.125 mM) showed meiotic restart and reduced cAMP expression as db-cAMP concentration fell. db-cAMP increases cAMP and cGMP levels in cultured oocytes, improving nuclear and cytoplasmic maturation and slowing ageing [6,9]. Thus, 1 mM db-cAMP may reduce spontaneous meiotic resumption, oocyte ageing, and oocyte growth in diverse ART programmes. Thus, 1 mM db-cAMP increased intraoocyte cyclic nucleotides, causing a complete meiotic halt. Reduced intraoocyte cAMP and cGMP levels in control oocytes promote cAMP-phosphodiesterase 3A (PDE 3A) [44, 45]. A decrease in intraoocyte cAMP may destabilise MPF and cause meiotic resumption after M-I arrest and PB-I extrusion. A modest increase in ROS stimulates the meiotic cell cycle in vitro, while high amounts alter mitochondrial potential and induce apoptosis [46].
Our results showed that 1 mM db-cAMP treatment during oocyte maturation resulted in higher levels of intracellular reactive oxygen species (ROS) and Ca2+ in comparison with the control group. Previous research, however, has shown the importance of a modest rise in ROS and Ca2+ during the maturation of mammalian oocytes [24,47]. However, our findings support that increasing the culture duration of an oocyte in db-cAMP may result in a rise in reactive oxygen species (ROS) and Ca2+ levels [46, 48], suggesting that an oocyte cultivated in 1 mM of db-cAMP may be fertile if not incubated for more than 12 hours. If the concentration and culture duration are raised beyond 12 hours, mitochondrial damage may occur in the oocytes. In spite of this, we have conducted experiments showing that db-cAMP treatment may keep cells in meiotic arrest for 12-18 hours.
Conclusion
In conclusion, one of the biggest obstacles to in vitro embryo development is improving reproductive outcomes during oocyte maturation. When the oocyte is removed from the antral follicle for in vitro maturation (IVM), cAMP levels inside the oocyte decrease, and meiosis restarts on its own owing to a lack of inhibitory chemicals in the follicle. Increased cAMP concentrations prior to IVM have been shown to enhance oocyte competence and, by extension, embryonic development in a number of species. Our results show that mammalian oocytes can be maintained in meiotic arrest (M-I stage) for up to 12 hours following treatment with 1 mM db-cAMP without compromising their viability, mitochondrial distribution, or developmental competence during in vitro culture. Since db-cAMP plays a critical function in regulating many oocyte development factors during oocyte handling in in vitro fertilisation, greater investigation into this route is warranted.
Funding
This study was partially funded by Centre of Advanced Studies, Banaras Hindu University, Varanasi, UP and partially by Indian Council of Medical Research, New Delhi, India.
Ethical Approval
All procedures confirmed to the stipulations of the Departmental Animal Ethical Committee of Banaras Hindu University, Varanasi-221005 and followed the guidelines for the care and use of laboratory animals (NIH Publication). All studies were in confirmation to the terms of Institutional Animal Ethical Committee (Ref. No.- BHU/DoZ/IAEC/2018-19/013) of the university.
Author’s Contributions
Alka Sharma: Conceptualization, Methodology, Software, Data curation, Writing - original draft, Visualization, Investigation. Pawan K Dubey and Anima Tripathi: Coordinated the experiments, Writing - review & editing and approved the final manuscript.
Declarations
The authors declare that they have no conflicts of interest.
References
ol>- Filatov MA, Khrsamova YV, Semenova ML (2015) In Vitro Mouse Ovarian Follicle Growth and Maturation in Alginate Hydrogel: Current State of the Art. Acta Naturae 7(2): 48-56.
- Filatov M, Khrsamova Y, Semenova M (2019) Molecular Mechanisms of Prophase I Meiotic Arrest Maintenance and Meiotic Resumption in Mammalian Oocytes. Reproductive Science 26: 1519-1537.
- Bagg MA, Nottle MB, Grupen CG, Armstrong DT (2006) Effect of dibutyryl cAMP on the cAMP content, meiotic progression, and developmental potential of in vitro matured pre-pubertal and adult pig oocytes. Molecular Reproduction and Development 73(10): 1326-1332.
- Duncan FE, Moss SB, Williams CJ (2006) Knockdown of the cAMP-dependent protein kinase (PKA) Type I alpha regulatory subunit in mouse oocytes disrupts meiotic arrest and results in meiotic spindle defects. Developmental dynamics 235(11): 2961-2968.
- Kirschner LS, Yin Z, Jones GN, Mahoney E (2009) Mouse models of altered protein kinase A signaling. Endocrine-related cancer 16(3): 773-793.
- Guixue Z, Luciano AM, Coenen K, Gandolfi F, Sirard MA (2001) The influence of cAMP before or during bovine oocyte maturation on embryonic developmental competence. Theriogenology 55(18): 1733-1743.
- Gupta A, Chaube SK (2020) Cilostamide and rolipram prevent spontaneous meiotic resumption from diplotene arrest in rat oocytes cultured in vitro. European Journal of Pharmacology 878: 173115.
- Conti M, Hsieh M, Zamah AM, Oh JS (2012) Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Molecular Cellular and Endocrinology 356(1-2): 65-73.
- Cheon YP, Kim SW, Kim SJ, Yeom YI, Cheong C, et al. (2000) The role of RhoA in the germinal vesicle breakdown of mouse oocytes. Biochemical and biophysical research communications 273(3): 997-1002.
- Fujii J, Iuchi Y, Okada F (2005) Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reproductive Biology and Endocrinology 3: 43-52.
- Kubiak JZ, Ciemerych MA, Hupalowska A, Sikora-Polaczek M, Polanski Z (2008) On the transition from the meiotic to mitotic cell cycle during early mouse development. The International journal of developmental biology 52(2-3): 201-217.
- Tiwari M, Gupta A, Sharma A, Prasad S, Pandey AN, et al. (2018) Role of Mitogen Activated Protein Kinase and Maturation Promoting Factor During the Achievement of Meiotic Competency in Mammalian Oocytes. Journal of Cellular Biochemistry 119(1): 123-129.
- Sharma A, Tiwari M, Gupta A, Pandey AN, Yadav PK, et al. (2018) Journey of oocyte from metaphase-I to metaphase-II stage in mammals. Journal of Cellular Physiology 233(8): 5530-5536.
- Tiwari M, Chaube SK (2017) Maturation promoting factor destabilization mediates human chorionic gonadotropin induced meiotic resumption in rat oocytes. Development Growth & Differentiation 59(7): 603-614.
- Nabti I, Reis A, Levasseur M, Stemmann O, Jones KT (2008) Securin and not CDK1/cyclin B1 regulates sister chromatid disjunction during meiosis II in mouse eggs. Developmental Biology 321(2): 379-386.
- Prasad S, Koch B, Chaube SK (2016) Maturation promoting factor destabilization facilitates postovulatory aging-mediated abortive spontaneous egg activation in rat. Development Growth & Differentiation 58(3): 293-302.
- Tripathi A, Chaube SK (2012) High cytosolic free calcium level signals apoptosis through mitochondria-caspase mediated pathway in rat eggs cultured in vitro. Apoptosis 17: 439-448.
- Mehlmann LM (2005) Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 130(6): 791-799.
- Izadyar F, Zeinstra E, Bevers MM (1998) Follicle-stimulating hormone and growth hormone act differently on nuclear maturation while both enhance developmental competence of in vitro matured bovine oocytes. Molecular Reproduction and Development 51(3): 339-345.
- Kuran M, Hutchinson JS, Broadbent PJ (1996) The response of bovine granulosa cells to different gonadotrophins in culture. Animal reproduction science 45(1-2): 1-12.
- Appeltant R, Beek J, Vandenberghe L, Maes D, Van Soom A (2015) Increasing the cAMP concentration during in vitro maturation of pig oocytes improves cumulus maturation and subsequent fertilization in vitro. Theriogenology 83(3): 344-352.
- Aktas H, Wheeler MB, First NL, Leibfried-Rutledg ML (1995) Maintenance of meiotic arrest by increasing [cAMP]i may have physiological relevance in bovine oocytes. Journal of Reproduction and Fertility 105(2): 237-245.
- Mattioli M, Galeati G, Barboni B, Seren E (1994) Concentration of cyclic AMP during the maturation of pig oocytes in vivo and in vitro. Journal of Reproduction and Fertility 100(2): 403-409.
- Tripathi A, Pandey V, Sahu AN, Singh A, Dubey PK (2019) Di-(2-ethylhexyl) phthalate (DEHP) inhibits steroidogenesis and induces mitochondria-ROS mediated apoptosis in rat ovarian granulosa cells. Toxicology research (Camb) 8(3): 381-394.
- Pandey V, Tripathi A, Rani A, Dubey PK (2020) Deoxyelephantopin, a novel naturally occurring phytochemical impairs growth, induces G2/M arrest, ROS-mediated apoptosis and modulates lncRNA expression against uterine leiomyoma. Biomedicine & Pharmacotherapy 131: 110751.
- Tripathi A, Pandey V, Sahu AN, Singh AK, Dubey PK (2019) Encircling granulosa cells protect against di-(2-ethylhexyl) phthalate-induced apoptosis in rat oocytes cultured in vitro. Zygote 27(4): 203-213.
- Wu XC, Han Z, Hao X, ZhaoYT, Zhou CJ, et al. (2020) Combined use of dbcAMP and IBMX minimizes the damage induced by a long-term artificial meiotic arrest in mouse germinal vesicle oocytes. Molecular Reproduction and Development 87(2): 262-273.
- Paschoal DM, Maziero RRD, Sudano MJ, Guastali MD, Vergara LE, et al. (2015) In vitro embryos production after oocytes treatment with forskolin. Zygote 24(2): 161-71.
- Adhikari D, Liu K (2014) The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Molecular and Cellular Endocrinology 382(1): 480-487.
- Gilchrsist RB (2011) Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reproduction Fertility and Development 23(1): 23-31.
- Sahu K, Gupta A, Sharma A, Tiwari M, Sahu K, et al. (2018). Role of granulosa cell mitogen-activated protein kinase 3/1 in gonadotropin-mediated meiotic resumption from diplotene arrest of mammalian oocytes. Growth Factors 36(1-2): 41-47.
- Tiwari M, Prasad S, Tripathi A, Pandey AN, Ali I, et al. (2015) Apoptosis in mammalian oocytes: a review. Apoptosis 20(8): 1019-1025.
- Zhao MH, Jin YX, Lee SK, Kim NH, Cui XS (2014) Artificial control maturation of porcine oocyte by dibutyryl cyclic AMP. Animal cells and systems 18(1): 52-58.
- Madgwick S, Jones KT (2007) How eggs arrest at metaphase II: MPF stabilization plus APC/C inhibition equals Cytostatic Factor. Cell Division 2(4).
- Premkumar KV, Chaube SK (2013) An insufficient increase of cytosolic free calcium level results postovulatory aging-induced abortive spontaneous egg activation in rat. Journal of Assisted Reproduction and Genetics 30(1): 117-123.
- Tiwari M, Chaube SK (2017) Reduction of nitric oxide level results in maturation promoting factor destabilization during spontaneous meiotic exit from diplotene arrest in rat cumulus oocytes complexes cultured in vitro. Development growth & differentiation 59(7): 615-625.
- Downs SM (2010) Regulation of the G2/M transition in rodent oocytes. Molecular Reproduction and Development 77(7): 566-585.
- Zhang M, Xia G (2012) Hormonal control of mammalian oocyte meiosis at diplotene stage. Cellular and Molecular Life Science 69(8): 1279-1288.
- Sharma A, Gupta A, Tiwari M, Sahu K, Prasad S, et al. (2018) Oocyte Quality and Female Infertility. Global Journal of Reproductive Medicine 3(2): 555607.
- Wassarman PM, Albertini DF (1994) From the mammalian ovum. In: Knobil E, Neill JD, editors. The physiology of reproduction Volume 1; 2nd New York: Raven-Press, pp. 79-122.
- Egbert JR, Uliasz TF, Shuhaibar LC, Geerts A, Wunder F, et al. (2016). Luteinizing Hormone Causes Phosphorylation and Activation of the cGMP Phosphodiesterase PDE5 in Rat Ovarian Follicles, Contributing, Together with PDE1 Activity, to the Resumption of Meiosis. Biology of Reproduction 94(5): 110.
- Wang Y, Teng Z, Li G, Mu X, Wang Z, et al. (2015) Cyclic AMP in oocytes controls meiotic prophase I and primordial folliculogenesis in the perinatal mouse ovary. Development 142(2): 343-351.
- Tiwari M, Chaube SK (2016) Moderate increase of reactive oxygen species triggers meiotic resumption in rat follicular oocytes. Journal of Obstetrics and Gynecology Research 42(5): 536-546.
- Basini G, Simona B, Santini SE, Grasselli F (2008) Reactive oxygen species and anti-oxidant defences in swine follicular fluids. Reproduction Fertility and Development 20(2): 269-274.
- Liu X, Wang J, Zhou M, Dai Q, Wang Q, et al. (2020) Particulate matter exposure disturbs inflammatory cytokine homeostasis associated with changes in trace metal levels in mouse organs. Science total environment 727: 138377.






























