Abstract
Purpose: Chronic rhinosinusitis with nasal polyps (CRSwNP) present different endotypes. There is evidence that patients from Brazil may present with a different endotype than western countries, with a predominantly mixed pattern of inflammation. Biomarkers may be used to identify patients with poor prognosis. The objective of our study is to measure tissue and serum eosinophils in patients with CRSwNP and to evaluate the correlation of serum and tissue eosinophils, as well as the correlation of serum, tissue eosinophils and asthma with recurrence and time to recurrence (<12 months) of nasal polyps.
Methods: Overall, 143 patients with chronic rhinosinusitis with nasal polyps who were submitted to endoscopic sinus surgery between 2009 to 2019 were enrolled in this study. It was analyzed correlation between tissue and serum eosinophilia, concordance between both markers and analysis of nasal polyp recurrence.
Results: We observed a higher median in the tissue eosinophils infiltrate among our patients when compared to the literature. We also identified correlation between asthma (p=0.027) and eosinophilia (p=0.003) with early recurrence of nasal polyps. There was no correlation between tissue and serum eosinophilia.
Conclusion: The patients of our study presented with a higher eosinophil infiltrate; however, the results did not demonstrated concordance of the tissue eosinophils with serum eosinophils nor correlation with polyp relapse. Patients with asthma and high serum eosinophilia also presented a higher risk of short term (12 months) polyp relapse. They may be utilized as biomarkers of poor prognosis in a population with mixed inflammation pattern.
Keywords:Chronic rhinosinusitis; Nasal polyps; Biomarker; Type 2 inflammation
Abbreviations:CRSwNP: Chronic Rhinosinusitis With Nasal Polyps; CRS: Chronic Rhinosinusitis; IgE: immunoglobulin E; eCRS: Eosinophilic Chronic Rhinosinusitis
Introduction
Chronic rhinosinusitis (CRS) was initially divided into two phenotypes: CRSwNP and rhinosinusitis without nasal polyps (CRSsNP). This classification has long guided the treatment and prognosis of chronic rhinosinusitis. In 2006, Van Zele et al. [1] demonstrated that these phenotypes responded to distinct immunological patterns, with CRSwNP usually presenting a type 2 pattern. CRS with Th2 endotype presents eosinophilic infiltrate, increased interleukins IL-4, IL-5, IL-13, IL-33, IL-25, eotaxin, eosinophilic cationic protein, and immunoglobulin E (IgE) [2]. It represents up to 85% of western patients with chronic rhinosinusitis with polyps [3]. It seems relevant to emphasize that the recurrence of polyps after surgical treatment can reach up to 60% over a period of 10 years [4], with up to 40% of patients presenting recurrence within a period of less than 12 months [5].
Fokkens et al. [6], in the most recent European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS, 2020), classified the diffuse chronic rhinosinusitis into eosinophilic chronic rhinosinusitis (eCRS) and non-eosinophilic chronic rhinosinusitis. Such definition is determined by the count of the number of eosinophils, as defined by the EPOS 2020 panel as ≥10 eosinophils in the high-power field (eos/HPF-400x). However, there is no consensus regarding the criteria for defining eCRS [7]. Furthermore, there is evidence that patients from our country may present with a different endotype, with a predominantly mixed pattern of inflammation [8]. In the light of these recent findings, the objective of this study is to measure tissue and serum eosinophils in patients with CRSwNP and to evaluate the correlation of serum and tissue eosinophils, as well as the correlation of serum, tissue eosinophils and asthma with recurrence and time to recurrence (<12 months) of nasal polyps.
Materials and Methods
Our retrospective study included patients with chronic rhinosinusitis with polyps, who underwent surgery between 2009 and 2019 at our institution. The participating patients were initially treated clinically and when there was no clinical response, they were referred for surgical treatment according to EPOS guidelines. Patients presenting eosinophilic granulomatosis with polyangiitis, primary ciliary dyskinesia, cystic fibrosis, immunodeficiency or neoplasia, and patients who had less than 1 month of postoperative follow-up were excluded. Clinical data such as gender, age, presence of asthma, presence of sensitivity to non-steroidal anti-inflammatory drugs, presence of recurrence, time to recurrence after surgery, and serum eosinophilia were obtained. Biopsies were performed in the office setting with a washout period from corticosteroids (oral and topical) of four weeks. Standard techniques were used to prepare all samples. The microscopic review was performed by two independent observers using a binocular microscope (Leica DM4000B LED, Switzerland). The observers were blinded to the clinical data and characteristics of the patients. Initially, the areas with the greatest subepithelial infiltrate were identified and, subsequently, examined in the high-power field (HPF-400x). The three fields with the highest infiltration were selected and photographic documentation was carried out.
Five photos of the area were taken in a standardized way for all patients in the sample, which determined an area compatible with the HPF area of the microscope used (0,333mm2). Afterwards, the eosinophils were counted and the average of the 3 areas was obtained. Patients were treated postoperatively with nasal irrigation with saline solution and topical nasal corticosteroids (budesonide or mometasone). Follow-ups were carried out with clinical evaluation and nasal endoscopy. The study was approved by the local ethical committee of the Campinas University Hospital (53910621.5.0000.5404) and an informed consent was obtained from every patient.
Statistical Analysis
Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) software (IBM SPSS Statistics for Macintosh, Version 28.0.). In the event of missing data, the characteristics of patients who had less than 40% missing data were inserted in the database using the XLSTAT Statistical Software for Excel. For qualitative (categorical) data, the missing data were estimated using the NIPALS (Nonlinear Iterative Partial Least Squares) algorithm, while for quantitative (numerical) data, missing data were estimated using the MCMC (Markov Chain Monte Carlo) multiple imputation algorithm. The Spearman correlation test was used in the comparison between groups and the significance level was set at alfa =0,05.
Results
One hundred and forty-three patients were included in the study, most of them were men (59.4%) and their average age was 50 years. Other relevant data found included the fact that the prevalence of asthma among participants was 39.1% and patients with aspirin exacerbated respiratory disease represented 11.9% of those included in the study (Table1). The serum eosinophilia median was 360 eosinophils/mm3 (eos/mm3) and the tissue eosinophils median count was 234 eos/HPF (Table 2). Considering the cutoff point proposed in the latest EPOS, we observed that 90% of the population might be considered to have chronic sinusitis with eosinophilic predominance. Sixty-nine patients (48.5%) presented recurrence and, out of them, thirty-five (50%) developed it before 12 months of follow-up. The mean time for nasal polyp relapse was 18.6 months. No variable was correlated with polyp recurrence. However, the Spearman test showed that patients with asthma (p<0.027) and serum eosinophilia >300 eos/mm3 (p<0.003) presented greater chances of recurrence before 12 months of follow-up (Table 3 & Table 4).
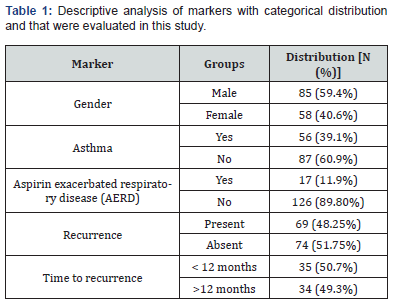
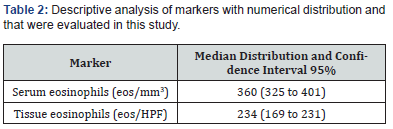
Eos, eosinophils; HFP, high-power field.
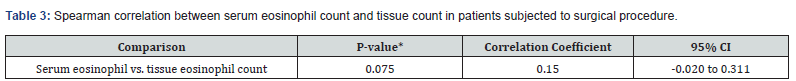
95% CI, 95% confidence interval.
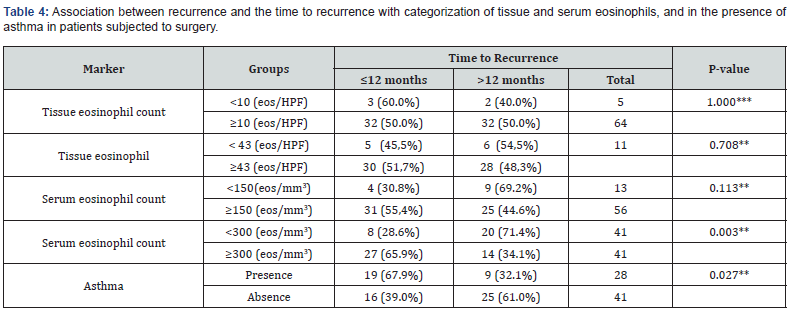
Eos, eosinophils; HFP, high-power field.
Discussion
CRS is an illness that affects between 5% and 12% of the global population [6]. Despite great advances in the understanding of immunological pathways [1-3], chronic rhinosinusitis is still a complex disease, with several endotypes and heterogeneous clinical results. In a recent study, Calus [9] reported that up to 80% of the patients might present polyp recurrence in the long term, and around 36% of them might require revision surgery. This data confirms the results obtained by van der Veen et al. [10], which demonstrated that 40% of the patients did not have control of their disease based on the EPOS control guidelines. Classically, tissue eosinophils count has been used as the type 2 inflammation biomarker CRS. However, lack of consensus about the cutoff point [7] hampers the comparison of data from different regions or institutions. Our study demonstrated a population with type 2 inflammation like that found in the literature [9], representing 90% of the sample. However, the median tissue eosinophils (234 eos/HPF) of our sample were much higher than that of other similar studies developed with western populations [11,12].
Other authors [13] had previously suggested that the cutoff point widely used (≥10 eos/HPF) might not represent properly the eCRS. In fact, great heterogeneity is found in the studies [7], most did not describe how many researchers carried out the histopathological analysis and about half of them did not mention the number of high-power fields used in the count of their area. Also, the area of the HPF reviewed and the use of graduated reticle mounted within the eyepiece objectives are not standardized in the literature. Other aspect to be explored in the difference of the tissue eosinophil count is geographical and ethnic differences in the CRS endotypes [14]. Our population presents a distinct ethnic conformation due to its diversity. Recently, Romano et al. [8] reported that in a multi-institutional study from Brazil most of the patients with CRSwNP presented with a mixed inflammation pattern demonstrated with a high concentration of cytokines from type 1,2 and 3 pattern. There was no difference in cytokines concentration when utilized the EPOS cut-off point (>10 eos/HPF) in the patients of the study. Therefore, it was proposed a cutoff point (≥43 eos/HPF) according to the cluster analysis of the patients based on the cytokines concentration.
The interest in serum eosinophil as a type 2 marker in chronic rhinosinusitis was recently renewed [15,16], mainly for its use in the indication of immunobiological drugs such as it is used in asthma. Previously, Tokunaga et al. [17] and Sakuma et al. [18] had already proposed the inclusion of serum eosinophilia and asthma in their models for the eCRS definition. However, the correlation between serum and tissue eosinophils remains unclear, and conflicting results are found in the literature [19,20]. Our results pointed out that there was no correlation between serum and tissue eosinophils in the patients of the sample. This may indicate that different endotypes could have different correlations between serum and tissue eosinophils due to different patterns of inflammation. The use of polyp recurrence is one of the main markers of severity and the presence of the type 2 inflammation in CRS.
The literature has recently demonstrated that asthma patients [21] and increased serum eosinophilia [16] tend to present a worse prognosis with increased risk of polyp recurrence and reoperation. Our results agree with these conclusions, with serum eosinophilia ≥300 (eos/mm3) (p<0.003) and the presence of asthma (p<0.027) indicating early recurrence (<12 months of follow-up). Moreover, they also indicate that tissue eosinophil count from our population might be closer to the reported by Asian authors [17,22] than to those presented by western authors [23,24]. However, there was no correlation between recurrence of nasal polyps with the eosinophil infiltrate. This study has some limitations such as the inclusion of a convenience sample from a referral university hospital, which might prevent the generalization of the results obtained in relation to other research centers. The sample size was relevant considering other studies published, however, limited in relation to statistical power. Moreover, since this is a retrospective study, the postoperative follow-up showed some chronological variation. Thus, conclusions based on the findings must be considered with caution.
Conclusion
The chronic rhinosinusitis is a challenging disease with multiple endotypes and high percentage of recurrence and reoperation. The patients of our study presented with a higher eosinophil infiltrate; however, the results did not demonstrated concordance of the tissue eosinophils with serum eosinophils nor correlation with polyp relapse. Patients with asthma and high serum eosinophilia also presented a higher risk of short term (12 months) polyp relapse. They may be utilized as biomarkers of poor prognosis in a population with mixed inflammation pattern.
Acknowledgment
We are extremely grateful to the whole Anatomical Pathology Department of the University of Campinas for its technical support. We also thank Prof. Fernando Marson for reviewing the manuscript and statistical advice.
References
- Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G (2006) Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61: 1280-1289.
- Gevaert P, Han JK, Smith SG, Sousa AR, Howarth PH (2022) The roles of eosinophils and interleukin-5 in the pathophysiology of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol 12(11):1413-1423.
- Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J (2016) Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol 137(5): 1449-1456.
- Riva C, Tavassoli M, Cravero E, Moresco M, Albera A (2022) Long-term evaluation of nasal polyposis recurrence: A focus on multiple relapses and nasal cytology. Am J Otolaryngol 43(2): 103325.
- DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL (2017) Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope 127(3): 550-555.
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R (2020) European Position Paper on Rhinosinusitis and Nasal Polyps. Eur Position Pap Rhinosinusitis Nasal Polyps. Rhinology 29: 37.
- Toro MDC, Antonio MA, Alves Dos Reis MG, De Assumpcao MS, Sakano E (2021) Achieving the best method to classify Eosinophilic Chronic Rhinosinusitis: a systematic review. Rhinology 59(4): 330-339.
- Romano FR, Valera FCP, Fornazieri MA, Lopes NMD, Miyake MM (2024) Inflammatory Profile of Chronic Rhinosinusitis With Nasal Polyp Patients in Brazil: Multicenter Study. Otolaryngol Head Neck Surg 171(5): 1552-1561.
- Calus L, Van Bruaene N, Bosteels C, Dejonckheere S, Van Zele T (2019) Twelve-year follow-up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin Transl Allergy 9: 30.
- Van der Veen J, Seys SF, Timmermans M, Levie P, Jorissen M (2017) Real-life study showing uncontrolled rhinosinusitis after sinus surgery in a tertiary referral centre. Allergy 72(2): 282-290.
- Bhattacharyya N, Vyas DK, Fechner FP, Gliklich RE, Metson R (2001) Tissue Eosinophilia in Chronic Sinusitis: Quantification Techniques. Arch Otolaryngol Head Neck Surg 127(9): 1102–1105.
- Brescia G, Alessandrini L, Parrino D, Franz L, Barion U (2019) Emerging Contribution of Histopathology to Our Understanding of Chronic Rhinosinusitis Endotypes: Tissue Eosinophil Count and Aggregates. Am J Rhinol Allergy 34(1): 122-126.
- Bassiouni A, Ou J, Rajiv S, Cantero D, Vreugde S (2016) Subepithelial inflammatory load and basement membrane thickening in refractory chronic rhinosinusitis with nasal polyposis: a histopathological study. Int Forum Allergy Rhinol 6(3): 248-55.
- Wang X, Zhang N, Bo M, Holtappels G, Zheng M (2016) Diversity of Cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol 138(5): 1344-1353.
- Brescia G, Barion U, Zanoni C, Giacomelli L, Martini A (2017) The prognostic role of serum eosinophil and basophil levels in sinonasal polyposis. Int Forum Allergy Rhinol 7: 261-267.
- Wang X, Meng Y, Lou H, Wang K, Wang C (2021) Blood eosinophil count combined with asthma history could predict chronic rhinosinusitis with nasal polyp recurrence. Acta Oto-Laryngologica 141(3): 279-285.
- Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S (2015) Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 70(8): 995-1003.
- Sakuma Y, Ishitoya J, Komatsu M, Shiono O, Hirama M (2011) New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus Larynx 38(5): 583-588.
- Gitomer SA, Fountain CR, Kingdom TT, Getz AE, Sillau SH (2016) Clinical Examination of Tissue Eosinophilia in Patients with Chronic Rhinosinusitis and Nasal Polyposis. Otolaryngol Head Neck Surg 155(1): 173-178.
- Aslan F, Altun E, Paksoy S, Turan G (2017) Could Eosinophilia predict clinical severity in nasal polyps? Multidiscip Respir Med 12: 21.
- Tsuzuki K, Hashimoto K, Okazaki K, Nishikawa H, Sakgami M (2019) Predictors of disease progression after endoscopic sinus surgery in patients with chronic rhinosinusitis. J Laryngol 133: 678-684.
- Lou H, Meng Y, Piao Y, Wang C, Zhang L (2015) Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy 29: 350-356.
- Kountakis SE, Arango P, Bradley D, Wade ZK, Borish L (2004) Molecular and cellular staging for the severity of chronic rhinosinusitis. Laryngoscope 114(11): 1895-905.
- Soler ZM, Sauer D, Mace J, Smith TL (2010) Impact of Mucosal Eosinophilia and Nasal Polyposis on Quality-of-Life Outcomes after Sinus Surgery. Otolaryngol Neck Surg 142(1): 64-71.





























