Abstract
Background: Acute Otitis Externa (AOE) is a common condition in ENT practice, often treated with ear packs containing topical medications and, in severe cases, oral antibiotics. Ichthammol glycerin is a frequently used topical medication in developing countries due to its cost-effectiveness and availability. Alternatively, antibiotic-steroid ear packs have been employed, and several studies have compared the effectiveness of these treatments. This systematic review evaluates the outcomes of ichthammol glycerin packs versus antibiotic-steroid ear packs.
Results: The review included eight full-text articles, six of which found that antibiotic-steroid ear packs generally led to better outcomes, particularly in reducing pain and promoting recovery. The remaining two studies did not show significant differences between the two treatments.
Conclusion: While the review supports the use of antibiotic-steroid ear packs, there are limitations to consider. The number of studies comparing the two treatments is relatively small, and the majority of research has been conducted in specific demographic groups, which may limit the generalizability of the findings. Therefore, further research with larger, more diverse sample sizes is needed to validate these results and offer a clearer understanding of the most effective treatment for AOE. This would help clinicians make more informed decisions when choosing the best therapeutic approach for their patients.
Keywords:Cochlear Implantation; Tonotopy; Frequency Range; Bandpass Filtering; Threshold Level; Frequency to Place Mismatch.
Abbreviations:AOE: Acute Otitis Externa; EAC: External Auditory Canal; IG: Ichthammol Glycerin; ARS: Numerical Rating Scale; VAS: Visual Analogue Score
Background
Acute Otitis Externa, also known as ‘Swimmer’s ear’ or ‘Tropical ear’ is defined as diffuse inflammation of the external ear canal which may also involve the pinna or the tympanic membrane. For otitis externa to be termed ‘acute’, the onset should generally be within 48 hours, in the past 3 weeks of symptoms and signs of ear canal inflammation [1]. The lifetime incidence of otitis externa is approximately 10% [2]. Acute Otitis Externa can present in two forms: Localized or Diffuse. Localized form presents with furunculosis in the ear canal. Diffuse form is the otitis externa mostly encountered in daily practice. The symptoms of ear canal inflammation are: Severe pain, itching, ear fullness, with or without hearing loss or jaw pain, intensified by jaw movement. On examination of the patient, there is tragal tenderness with pain on moving the pinna in all directions. Ear canal is swollen and edematous with or without regional lymph node inflammation [3]. A number of factors contribute to the etiology of otitis externa. These are: Aggressive cleaning of the ear, water exposure, trauma, irritant exposure, soapy deposits, sweating, wearing hearing aids, allergy, stress, etc. Warmer climates and humidity also play a role [4–7]. Cerumen is protective to the ear as it acts as a barrier to moisture and dust particles [8]. It has slightly acidic pH which helps in preventing infection but this is altered due to excessive cleaning and other etiological factors. This predisposes a patient to otitis externa.
Treatment of acute otitis externa consists of topical and systemic antimicrobial therapy with analgesics [1]. Topical antimicrobial therapy is administered via ear packs which act by its chemical constituent as well as splinting action of the edematous tissue. Of the various chemical compounds used, 10% Ichthammol glycerine has been used traditionally for ear packing. Ichthammol has antiseptic action and glycerine has hygroscopic action. This combination has anti-staphylococcal action [9,10]. Antibiotic steroid ointment is also used frequently for ear packs. Antibiotic component of the ointment controls infection and the steroid component reduces edema by its action on the capillary wall tone of the ear canal. Numerous studies have been conducted to compare the effectiveness between ear packs. The studies are mostly randomized with various parameters used to measure outcome [11-19]. This study aims to systematically review the outcomes of Ichthammol glycerin and antibiotic steroid ear packs in patients with otitis externa.
Objective
To perform a systematic review to compare the effectiveness of Ichthammol glycerin pack in comparison to antibiotic steroid pack as treatment for acute otitis externa.
Methods
The manuscript was prepared according to the guidelines outlined in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement, ensuring a systematic and transparent approach to conducting and reporting the systematic review. Furthermore, prior to conducting the systematic review, a detailed protocol was developed and registered in the International Prospective Register for Systematic Reviews (PROSPERO). The protocol registration is identified by the unique Registration Number CRD42023393549.
Literature search
An author conducted a comprehensive literature search spanning the years 2008 to 2021 across multiple databases, including PubMed, Scopus and Google scholar. This systematic exploration aimed to identify relevant studies, articles, and scholarly works pertinent to the research topic. The details of the search terms employed by the author can be found in Appendix 1.
Eligibility criteria
All the studies in which the patients were diagnosed as otitis externa and underwent packing with 10% Ichthammol glycerin pack and any form of Antibiotic steroid pack were included. Both randomized and non-randomized clinical trials were included. Studies which used only antibiotic or steroid ointment, topical drops application or comparison with other forms of treatment were excluded from the study (Chart 1).
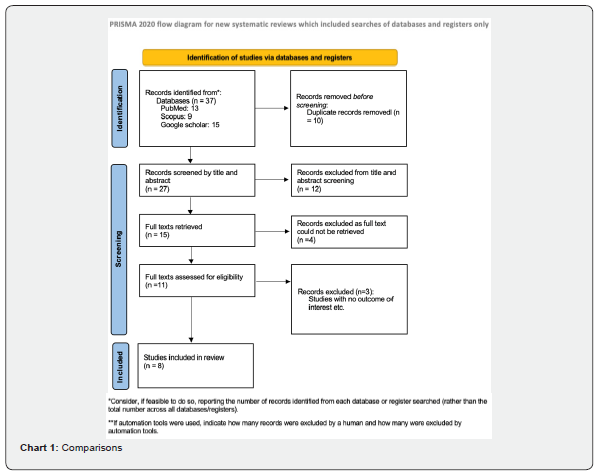
Data collection and analysis
Two authors independently searched using the search strategy in different databases. Studies written in languages other than English were excluded. Duplicate studies were then removed. The remaining articles were then screened from title and abstract. Studies not fitting the inclusion criteria were excluded. Full texts of the screened articles were retrieved. Then, all the full texts were reviewed one by one and those studies with relevant study characteristics and outcome were extracted for inclusion by two independent authors. Studies with no outcome of interest were excluded. If there was disparity in any step of this process, discussion was conducted among all the authors and final decision was made based upon majority’s decision.
Primary outcome
Pain was taken as the primary outcome. This was assessed after 48 hours of pack insertion.
Secondary outcome
a) Number of hospital visits: This was defined as number of follow up visits to the hospital until the pain subsided or there was no tragal tenderness.
b) EAC edema: This was taken as visible edema of the external auditory canal.
c) Debris/discharge: Presence or absence of debris and discharge was taken as a secondary outcome measure.
Risk of Bias within Studies
Risk of bias was assessed for all randomized control trial and non-randomized studies by two authors independently. For randomized control trial studies, Cochrane risk of bias tool for randomized trial version 2 (ROB 2) was used for assessment in 5 domains (randomization process, deviation from intended interventions, missing outcome data, measurement of outcome, selection of reported result), along with the overall risk of bias. For non-randomized study, ROBINS-I tool was used. If there were any disagreements during assessment, it was resolved by further discussion.
Summary Measures and Synthesis of Results
Information regarding the study (study details, country where study was conducted, aim, study design, sampling technique, sample size of population included and various outcome measures) were tabulated and a systematic narration was done. Risk of bias and quality of evidence were also summarized. GRADE (Grading of Recommendation, Assessment, Development and Evaluation) approach will be used to assess the certainty of the evidence for the eligible studies.
Decisions on meta-analysis were planned to be made on a consensus regarding the quality of evidence synthesized from systematic review. RevMan5 will be used to conduct meta-analysis on the basis of heterogeneity. Heterogeneity will be assessed by I square and accordingly, either fixed effect model or random effect model is planned to be used. Further, results will be calculated on the basis of the variable (dichotomous or continuous variable).
Results
Study selection and characteristics
We identified 37 studies from databases using the search strategy. All papers written in English language were included. 10 duplicate studies were then removed. 27 of the remaining studies were then screened for title and abstract. 12 articles were excluded after going through the title and abstract which was not in accordance with our inclusion criteria. Out of the 15 remaining articles, 11 full texts were found. These full texts were analyzed. 3 studies had no outcome of interest and were excluded. A critical review of the eight full-text articles included in the analysis is summarized in Table 1.
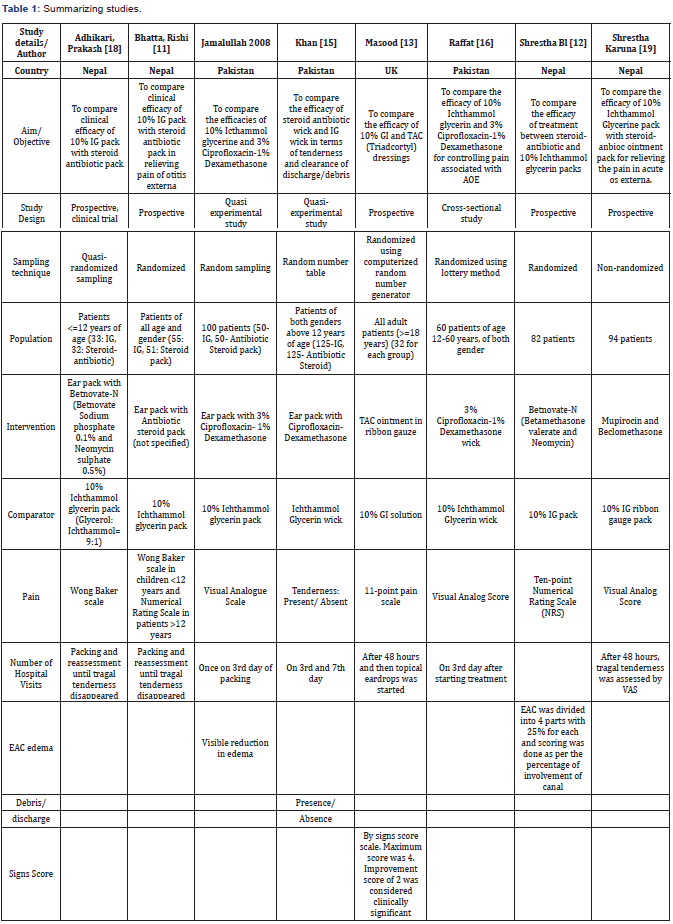
Primary outcomes
All the studies mentioned pain as their primary outcome measure. However, the scales used were different in different study as summarized in Table 2.
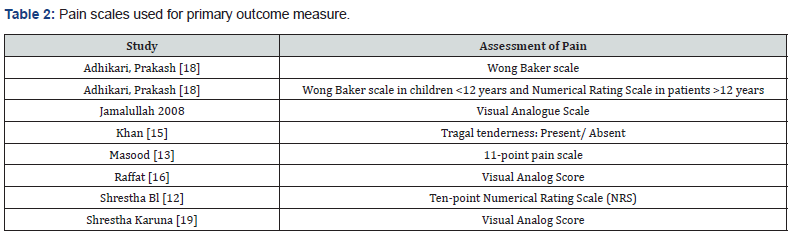
Secondary outcomes
Number of hospital visits: 7 out of 8 studies measured the number of hospital visits as the secondary outcome measure.
EAC edema: 2 studies measured the reduction in EAC edema. One study subjectively measured edema according to visible reduction and another study divided EAC into 4 parts with 25% for each and scoring was done as per the percentage of involvement of the canal.
Debris/discharge: Only one study took the presence or absence of debris and discharge as the outcome measure. A detailed analysis of the secondary outcome parameters utilized in each study is provided in Table 1 & Figure 1.
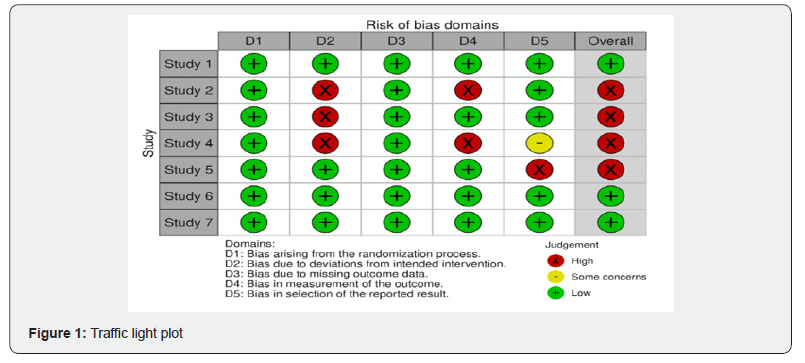
Discussion
Numerous studies have investigated the treatment of acute otitis externa, comparing various modalities, including topical antibiotic treatments, ear drops with or without steroids, polyurethane foam, boric acid, non-pharmaceutical methods, and oral antibiotics for severe cases [17,20-22]. Systematic reviews have previously compared antibiotic versus non-antibiotic treatments, but this is the first review to specifically compare ichthammol glycerin with an antibiotic-steroid ear pack [23]. A majority of the studies included in this review were conducted in Nepal and Pakistan, with only one study from a hospital in the UK. This disparity may be attributed to the more widespread use of ichthammol glycerin in developing countries, where it is considered a cost-effective and accessible treatment option.
Among the studies reviewed, one found no significant difference in clinical finding between ichthammol glycerin and the antibiotic-steroid ear pack. This study recommended ichthammol glycerin over the antibiotic-steroid ear pack, citing its cost-effectiveness, avoidance of resistance and toxicity. Another study observed improvements in pain relief with the use of the antibiotic-steroid wick, while also noting that ichthammol glycerin was equally effective in terms of overall efficacy and discharge reduction. However, the majority of studies reported significantly better outcomes with the antibiotic-steroid ear pack. Despite the promising findings, higher-quality studies are needed to strengthen the evidence base. Future research should include more diverse populations, larger sample sizes, and multiple outcome measures to better assess the comparative effectiveness of these treatments. Due to the use of different scales to measure outcomes across studies, we were unable to perform a meta-analysis. After careful consideration, all three authors agreed that conducting a meta-analysis would not provide meaningful insights for this review.
Conclusion
The analysis of the evidence revealed that antibiotic-steroid ear packs tended to produce better results, particularly in reducing pain and aiding recovery. However, the review also pointed out several limitations. The available studies comparing the two treatments are limited in number, and most of the research has been carried out on specific demographic groups, which restricts the broader applicability of the findings. Therefore, additional research with more diverse and larger participant groups is needed to confirm these results and provide a clearer insight into the most effective treatment for AOE.
References
- Rosenfeld RM, Schwartz SR, Cannon CR, Roland PS, Simon GR. Clinical Practice Guideline.
- Raza SA, Denholm SW, Wong JCH (1995) An audit of the management of acute otitis externa in an ENT casualty clinic. J Laryngol Otol 109(2): 130-133.
- Dibb WL (1991) Microbial aetiology of otitis externa. J Infect 22(3) :233-239.
- Jenkis BH (1963) Simplified approach to otitis externa. Arch Otolaryngol Chic Ill 77: 442-443.
- Martinez Devesa P (2025) External auditory canal pH in chronic otitis externa - Martinez Devesa - 2003 - Clinical Otolaryngology & Allied Sciences - Wiley Online Library.
- Russell JD, Donnelly M, Mcshane DP, Alun-Jones T, Walsh M (1993) What causes acute otitis externa? J Laryngol Otol 107(10): 898-901.
- (2025) Swimmers and Nonswimmers: Archives of Environmental Health: An International Journal 30(9).
- Nussinovitch M, Rimon A, Volovitz B, Raveh E, Prais D (2004) Cotton-tip applicators as a leading cause of otitis externa. Int J Pediatr Otorhinolaryngol 68(4): 433-435.
- Ahmed K, Roberts ML, Mannion PT (1995) Antimicrobial activity of glycerine-ichthammol in otitis externa. Clin Otolaryngol Allied Sci 20(3): 201-203.
- Nilssen E, Wormald PJ, Oliver S (1996) Glycerol and ichthammol: medicinal solution or mythical potion? J Laryngol Otol 110(4): 319-321.
- Bhatta R, Pokharel R, Adhikari P, Neupane Y (2009) A comparison of 10% Ichthalmmol Glycerine pack with steroid-antibiotic pack for relieving pain in cases of Acute otitis Externa. J Inst Med Nepal 31(1): 7-10.
- Shrestha BL, Shrestha I, Amatya RCM, Dhakal A (2010) Effective Treatment of Acute Otitis Externa: a Comparison of Steroid Antibiotic Versus 10% Ichthammol Glycerine Pack. Indian J Otolaryngol Head Neck Surg 62(4): 350-353.
- Masood A, Moumoulidis I, Ray S, Chawla O, Panesar J (2008) A randomised controlled trial comparing Triadcortyl® with 10% glycerine–ichthammol in the initial treatment of severe acute otitis externa. Eur Arch Otorhinolaryngol 265(8): 881-885.
- Di Traglia R, Tudor‐Green B, Muzaffar J, Borsetto D, Smith ME (2023) Antibiotics versus non‐antibiotic treatments for acute otitis externa: A systematic review and meta‐analysis. Clin Otolaryngol 48(6): 841-862.
- Khan M, Butt KAA, Riaz N, Hassan ZU, Ahmed A, Wasif M (2021) Comparison of the efficacy of antibiotic--steroid and ichthammol glycerine wick in treatment of acute otitis externa. PAFMJ 71(Suppl 3).
- Raffat Mustafa S (2019) Comparison of 3% Ciprofloxacin-1% Dexamethasone and 10% Ichthammol Glycerin for Control of Pain due to Acute Otitis Externa. J Islamabad Med Dent Coll 7(4): 260-264.
- Demir D, Yılmaz MS, Güven M, Kara A, Elden H (2018) Comparison of clinical outcomes of three different packing materials in the treatment of severe acute otitis externa. J Laryngol Otol 132(06) :523-528.
- Adhikari P, Bhatta R, Bhandari S, Pyakurel BM (2011) Comparison of steroid antibiotic pack and 10% ichthammol glycerine pack in relieving pain of acute otitis externa in children. Int J Pediatr Otorhinolaryngol 75(4): 500-503.
- Shrestha K, Shah R, Sapkota S (2019) Efficacy of 10% Ichthammol Glycerine Pack and Steroid-antibiotic Pack for Relieving Pain in Acute Otitis Externa. A Comparative Study. Birat J Health Sci 4(1): 634-638.
- Amani S, Moeini M (2016) Comparison of Boric Acid and Combination Drug of Polymyxin, Neomycin and Hydrocortisone (polymyxin NH) in the Treatment of Acute Otitis Externa. J Clin Diagn Res JCDR 10(7): MC01-MC04.
- Panahi Y, Akhavan A, Sahebkar A, Hosseini SM, Taghizadeh M (2014) Investigation of the effectiveness of Syzygium aromaticum, Lavandula angustifolia and Geranium robertianum essential oils in the treatment of acute external otitis: A comparative trial with ciprofloxacin. J Microbiol Immunol Infect 47(3): 211-216.
- Kantas I, Balatsouras DG, Vafiadis M, Apostolidou MTh, Pournaras A (2007) The use of trichloroacetic acid in the treatment of acute external otitis. Eur Arch Otorhinolaryngol 264(1): 9-14.
- Di Traglia R, Tudor‐Green B, Muzaffar J, Borsetto D, Smith ME (2023) Antibiotics versus non‐antibiotic treatments for acute otitis externa: A systematic review and meta‐analysis. Clin Otolaryngol 48(6): 841-862.





























