Treatment with Ozone Therapy for Osteomyelitis After Mandibular Fracture Fixation Failure - Clinical Case Report
Marina Pereira Silva1, Poliana Maria de Faveri Cardoso1, Rafael Da Silva Vanolli2, Natasha Magro Ernica3, Julio Katuhide Ueda4 and Veridiana Camilotti4*
1Master’s Student in Dentistry, State University of Western Paraná, Rua Universitária, Brazil
2Dentistry Student, State University of Western Paraná, Rua Universitária, Brazil
3DDS, MSc, Department of Oral and Maxillofacial Surgery and Traumatology, School of Dentistry, Brazil
4DDS, MSc, PhD, Department of Restorative Dentistry, School of Dentistry, Brazil
Submission: February 09, 2024; Published: February 28, 2024
*Corresponding author: Veridiana Camilotti, Department of Restorative Dentistry, Dental School, Rua Universitária, 2069 - Faculdade, Cascavel-PR, CEP 85.819-110, Brazil
How to cite this article: Marina Pereira Silva, Poliana Maria de Faveri Cardoso, Rafael Da Silva Vanolli, Natasha Magro Ernica, Julio Katuhide Ueda and Veridiana Camilotti*. Treatment with Ozone Therapy for Osteomyelitis After Mandibular Fracture Fixation Failure - Clinical Case Report. Glob J Oto, 2024; 26 (4): 556194. DOI: 10.19080/GJO.2024.26.556194
Abstract
Osteomyelitis, common in the jaw due to its limited blood supply, requires comprehensive treatment. Ozone therapy, recognized for its analgesic, anti-inflammatory, and antibacterial properties, stands out as a promising approach to bacterial disruption and modulation of inflammation. This study reports a clinical case of osteomyelitis resulting from a complex fracture of the mandible after a motorcycle accident. After complications with the initial fixation, ozone therapy was successfully applied in three sessions, using protocols associated with gas, water, and ozonized oil, with a seven-day interval between each session. This approach proved effective in treating the resulting chronic fistula. This report underlines the complexity of treating osteomyelitis, highlighting the effectiveness of ozone therapy, especially when associated with different protocols, as an innovative alternative for controlling infection and promoting healing.
Keywords: Osteomyelitis; Ozone; Anti-inflammatory; Staphylococcus; Cytokines
Introduction
In dentistry, osteomyelitis is a relatively rare pain-sensitive condition, most commonly seen in the mandible. It represents the main manifestation resulting from the action of Staphylococcus aureus, Streptococcus, Eikenella, and Candida, and its etiologies can result from bacterial dissemination from adjacent areas, direct inoculation of the microorganism or hematogenous dissemination [1]. Osteomyelitis, if not properly treated during the acute phase, can evolve into a chronic form with microorganisms that are more resistant to the pharmacological action of antibiotics (González-Navarro et al. 2017). In addition, patients can develop intermittent painful edema, facial asymmetry, facial fistulas, increased volume, and nerve paresthesias. In this context, the primary objective in resolving the case is to contain the infection, focusing on early diagnosis, drainage of pus, bacteriological culture accompanied by antibiotic sensitivity testing, appropriate antibiotic therapy, surgical debridement, and surgical reconstruction procedures [2].
Pharmacological treatment has been based on agents such as vancomycin, rifampicin, fusidic acid, minocycline, cotrimoxazole, and clindamycin, especially in cases of methicillin-resistant Staphylococcus aureus infections (Tuzuner-Oncul et al. 2009). Irrigation with povidone-iodine and chlorhexidine has proved effective, and rifampicin is also used as an antibiotic alternative for irrigation. However, the indiscriminate use of antibiotics can encourage the development of antibiotic-resistant bacteria [3]. Ozone, increasingly used in dentistry and medicine, is a triatomic oxygen compound and is generated from the transformation of medical oxygen using specific generators. Its gaseous form can alternate for oil or water [4]. Compared to oxygen, it is more dissolved in water, favoring its diffusion into tissues. It also has analgesic, inflammatory, and antibacterial properties [5]. In the bacterial context, the ozone molecule can induce rupture of the extracellular membrane, disrupting the enzyme regulatory system and amplifying membrane permeability, culminating in cell apoptosis.
Studies indicate its effectiveness even against antibioticresistant bacteria, without harming the cells of the human organism due to its antioxidant action [6]. In the immunological field, ozone acts to modulate the inflammatory system through the production of hydrogen peroxide, aldehydes, and lipid oxidation products. It also promotes the activation of erythrocyte nuclear factors (Nrf2), stimulating enzymes such as superoxide dismutase, catalase, and glutathione peroxidase [7]. In addition, ozone interferes with the NF-kB signaling pathway, exerting an anti-inflammatory effect by reducing the production of cytokines such as IL-1, IL-2, IL-6, and TNF-α. These cytokines, when deregulated, can impact the mineral homeostasis of bone tissue, influencing osteocyte proliferation and subsequent resorption [8]. In addition, ozone acts on red blood cells, increasing the efficiency of glycolysis, increasing the supply of oxygen to tissues, and generating free radical scavenging enzymes, mitigating oxidative stress [9].
Given the above, this case report highlights the complexity and challenges faced in treating osteomyelitis, a condition that can evolve into chronic forms, requiring innovative therapeutic approaches. The conventional protocol often involves the use of antibiotics, whose efficacy can be challenged by resistant microorganisms. In this context, this study describes the successful application of ozone therapy as a promising alternative for controlling infection and promoting healing. The results obtained, showing the resolution of the case after the application of the ozone therapy protocol, highlight not only the antimicrobial efficacy of ozone but also as well as its anti-inflammatory and immune system-modulating properties. It is hoped that this report will contribute to the understanding and consideration of innovative therapies in the management of complex cases of osteomyelitis, encouraging further research and improving the therapeutic options available.
Case Report
Anamnesis, clinical and radiographic examination
This is a clinical case of a 27-year-old male patient admitted to the Hospital Universitário do Oeste do Paraná in Cascavel, PR, after a motorcycle accident that resulted in fractures to the mandible and maxilla. The patient, a smoker, alcoholic, and cocaine user, denied any comorbidities, continuous use of medication, or allergies. Physical examination revealed no abnormalities in the upper third of the face. In the middle third, the patient had a right retro auricular hematoma, pain on right preauricular palpation, nasal abrasions, and preserved visual acuity and motility. In the lower third, there was a limitation in mouth opening of around 20mm, accompanied by a mandibular deviation to the right side due to an ipsilateral condylar fracture. Left submandibular edema was evident, and the patient had abrasions on the lips, left the malar region, and mandibular chin, with a laceration already sutured on the chin, with no signs of dehiscence, necrosis, or infection.
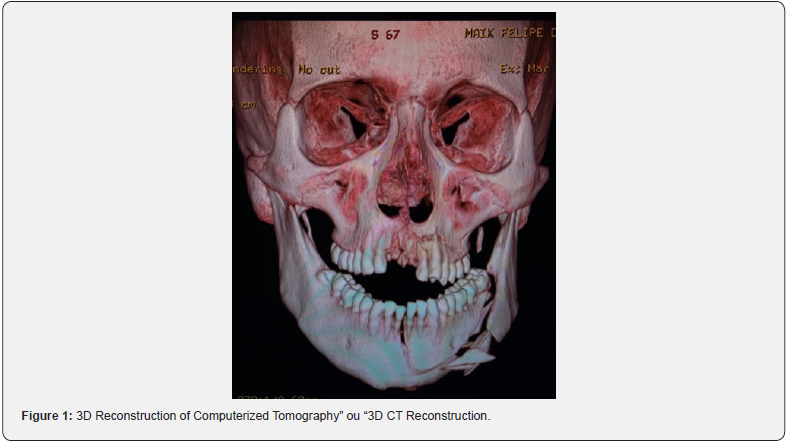
Intraoral examination revealed a laceration in the sulcus of the anterior region of the mandible, mobility of the bony stumps in the symphysis and left the mandibular body, altered occlusion with posterior premature touch on both sides and anterior open bite. There was dentoalveolar trauma with avulsion of teeth 11 and 21, coronal fracture of teeth 22 and 23, and paresthesia of the inferior alveolar nerve on the left. Computed tomography of the face showed fractures of the buccal bone plate in the left maxilla, a fracture of the right mandibular condyle, and a block fracture involving the symphysis and the left mandibular body, extending to the left mandibular angle (Figure 1).
Surgical procedure
Based on the anamnesis, clinical, and radiographic examinations, the decision was to wait for the edema to regress before surgery. The fractures in the mandibular symphysis and left mandibular body were accessed intraorally. After locating the fractures in these regions, a maxillo-mandibular block (MMB) was performed using locking screws (Allprime Select, São José- SC, Brazil) to aid reduction. After BMM and fracture reduction, the mandibular symphysis was fixed with two straight plates (Orthoface, Curitiba, PR, Brazil), one in the tension zone and the other in the compression zone. The left mandibular body was fixed with two straight plates. After fixation, copious irrigation and intraoral suturing with 4-0 vicryl (Johnson & Johnson Med Tech, São Paulo, SP, Brazil) were carried out. BMM with locking screws and heavy elastics was maintained for conservative treatment of the right mandibular condyle. Post-operative medication included cephalexin 500mg every 6 hours for 7 days, nimesulide 100mg every 12 hours for 3 days, and dipyrone 500mg/ml, 20 drops every 6 hours for 3 days. Mouthwash (chlorhexidine 0.12%) was also prescribed twice a day for 7 days.
Post-surgical follow-up
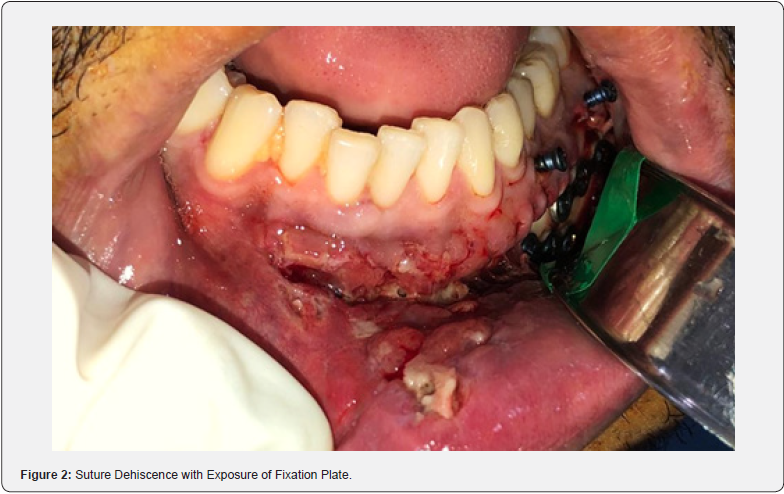
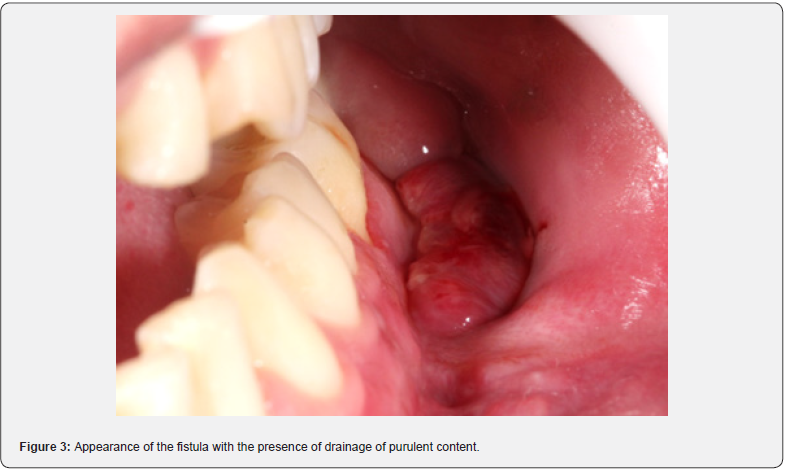
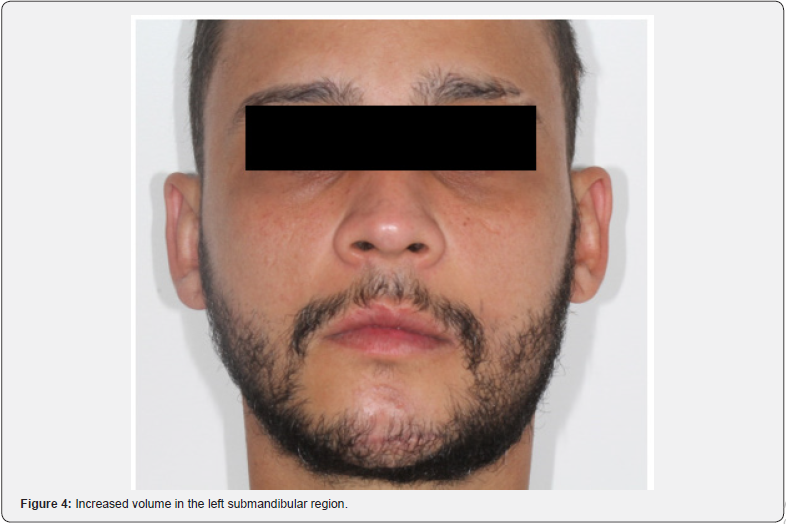
The post-operative CT scan shows bone stumps aligned with the osteosynthesis. After 7 days, the patient had edema, limited opening, and paresthesia of the left inferior alveolar nerve. In the intraoral assessment, suture dehiscence with necrotic margins and exposure of the upper plate in the left mandibular body is evident (Figure 2). Debridement, irrigation with 0.9% saline and 10% topical povidone iodine (PVPI), suturing with 4-0 nylon, reinsertion of elastic bands, and prescription of clindamycin and 0.12% chlorhexidine were carried out. After 13 days, a new dehiscence with infection required debridement, irrigation, and resuturing, with the reinsertion of elastic bands and clindamycin. Dehiscence with loss of substance persisted weekly. After 50 days, exposure of the upper plate and failure of the fixation led to removal without the need for replacement. After 14 days, new dehiscence was treated with irrigation, clindamycin, and chlorhexidine. After 28 days, the asymptomatic patient showed slow repair, a chronic fistula, purulent drainage (Figure 3), no pain, but an infectious focus, and left submandibular enlargement (Figure 4).
Ozone therapy
Given the persistence of the inflammatory condition, it was decided to start treatment with a protocol associated with ozone therapy. This protocol involved the application of medical ozone at a concentration of 20mcg/mL, together with the use of ozonized water and ozonized sunflower oil.
Production of ozonated water
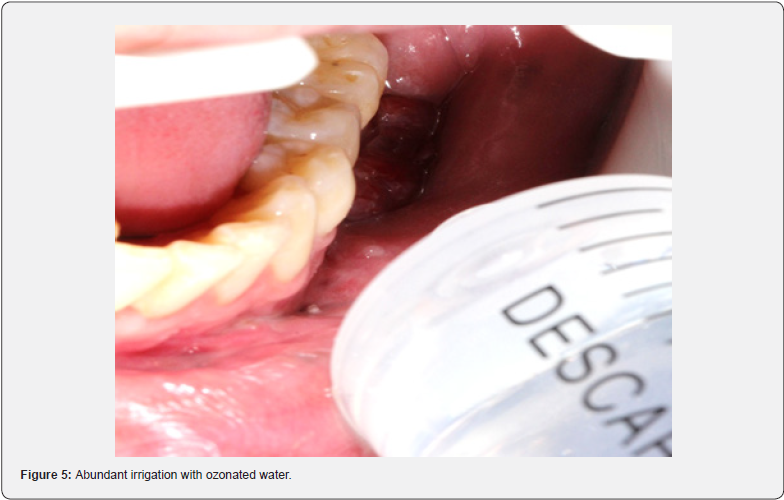
The 18 ppm ozonated water was prepared at an ambient temperature of 21ºC ± 1.0 ºC, five minutes ± 1.0 min before use, and used up to five minutes ±1.0 min after preparation. This was done using an ozone generator (Philozon Med plus V, Caboriu, SC, Brazil), which used pure oxygen from a cylinder attached to a glass tower (1L/min). The amount of ozone in the water was measured using direct iodometric titration, following the recommendations of the International Ozone Association (IOA). This method consists of adding 50ml of potassium iodide solution (KI)1N to previously ozonized water. The chemical reaction involved in this procedure resulted in the oxidation of KI by ozone, releasing iodine (I2), according to the equation O3 + 2 KI + H2O ↔ I2 + 2 KOH + O2. To ensure the production of I2, it was necessary to acidify the medium by adding 2.5ml of 1N sulfuric acid (H2SO4) to the KI solution. This was followed by titration with 0.01N sodium thiosulfate (Na2S2O3) until the yellowish color of the iodine became barely perceptible. Subsequently, 1ml of 1% starch indicator solution was added and titration was resumed until the blue color of the solution disappeared. For each session, 500ml of ozonized water was administered using a disposable syringe and Slim Microcannula (Fabinject, Taubaté, SP, Brazil) inside the fistula, as part of the debridement process (Figure 5).
Gas
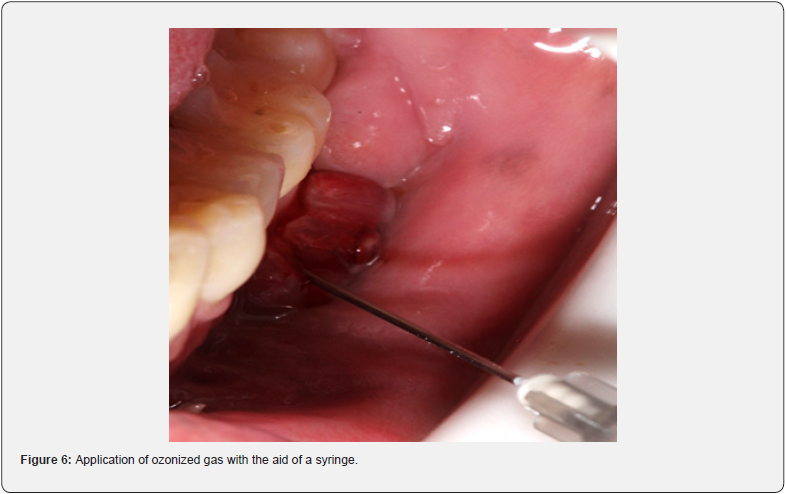
The ozonized gas was generated using an ozone generator (Philozon Med plus V, Caboriu, SC, Brazil) using medical oxygen. The generator was set to produce 20mcg/ml of gas. Subsequently, for each session, 3ml of this gas was applied inside the fistula, using a disposable syringe and an extra-short needle (Gingival Needle - AllPrime, São José - SC, Brazil), (Figure 6).
Ozonized sunflower oil
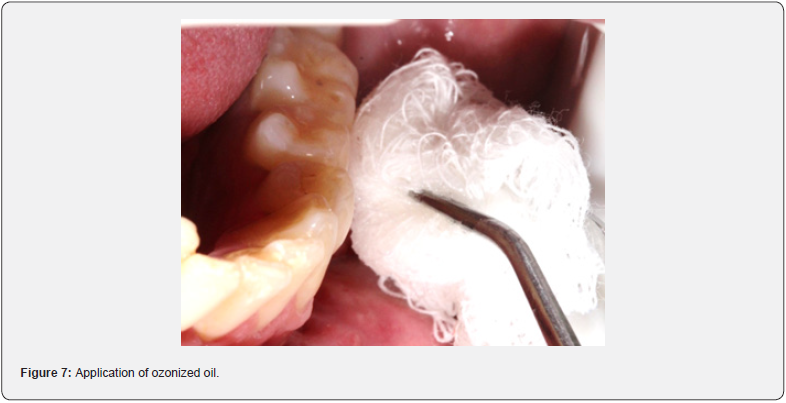
In this case, ozonized sunflower oil (Philozon, Caboriu, SC, Brazil) was applied to the fistula area using sterile gauze. In addition, the patient was instructed to apply the ozonized oil at home (Figure 7), twice a day, every day. During the ozone therapy treatment, the antibiotic the patient was taking was stopped.
Reassessment
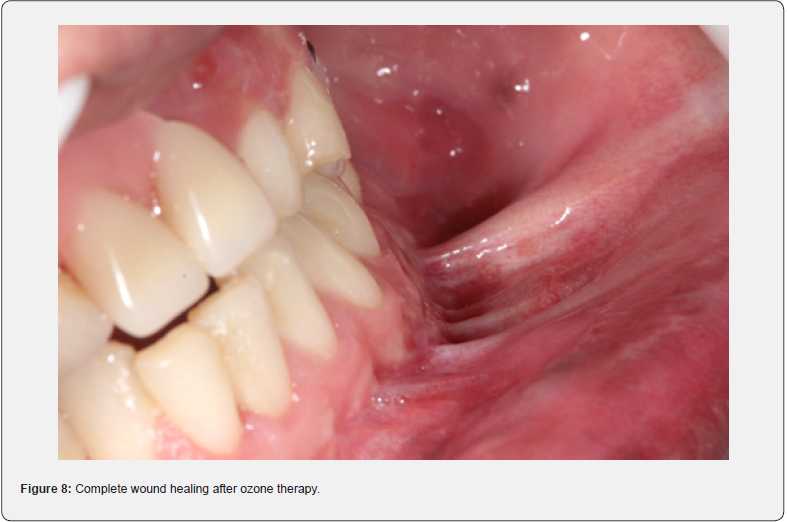
After three sessions of the associative protocol, the wound had healed completely (Figure 8) and the edema in the submandibular region on the left had regressed. The patient shows bone healing of the fractures, no painful symptoms, and no more infection, with complete regression of the fistula.
Discussion
Due to the presence of variables related to bone damage, such as the virulence factors of the microorganism, sensitivity to antibiotics, extent of the lesion, patient care, and harmful habits, such as smoking, alcohol consumption, self-medication, and frequent use of narcotics. The treatment of osteomyelitis is often challenging [10]. In this context, it is worth noting that the patient in this study also had harmful habits, such as alcohol and cocaine consumption, which can negatively influence the repair process.
In addition, classifications of bone infections do not have firmly established concepts, with the most widely accepted being the temporal criterion proposed by the studies by Willenegger & Roth in the 1980s [11]. According to these researchers, an infection that starts in less than two weeks is considered early, between two and ten weeks it is categorized as late, and after ten weeks it is characterized as late acute onset.
This temporal approach makes it easier to assess the severity of bone damage and cell degradation, simplifying the mechanisms of therapeutic intervention [11]. This temporal perspective also applies to the case in question, allowing for a more precise analysis of the chronology of the infectious condition and guiding treatment strategies. Osteoblasts, which originate from multipotent mesenchymal stem cells, play a central role in bone formation. As they mature, these cells turn into osteocytes, which in turn modulate cell movement. In contrast, osteoclasts are responsible for bone resorption [12]. The homeostasis of these cells is vital for bone health, and the regulation of calcium and phosphate levels is mediated by signaling pathways, including nuclear factor activating receptors, notably RANK and RANKL [13].
Nuclear factor binding (RANKL) establishes a connection with nuclear factor kappa B (NF-κB), initiating an intracellular signaling cascade that regulates immune responses. When activated, NF- κB migrates to the cell nucleus, influencing not only the immune response but also processes such as inflammation and cell survival [14]. This interaction is crucial for the activation of osteoclasts, playing a key role in bone remodeling. Osteoprotegerins (OPG) act as receptors that, when stimulated by RANKL, promote differentiation monocytes into osteoclasts, thus influencing bone density [15]. Under conditions of dysbiosis in bone tissue, the lipopolysaccharide (LPS) present in the outer membrane of bacteria triggers immune responses in the host. This results in increased expression of pro-inflammatory cytokines such as TNF-α, IL-6, IL-1, glucocorticoids, bradykinin, and histamine. These inflammatory mediators, in turn, affect the expression of RANK and RANKL, interrupting the bone remodeling process and impairing epithelial healing [16].
In this context, the wound-healing process becomes crucial to prevent the development of environments conducive to dysbiosis. This process is intricate and comprises not only chemotaxis but also cell division, neovascularization, scar remodeling, and the synthesis of extracellular matrix proteins [17]. In the present study, the patient had a complex fracture of the mandible, accompanied by significant damage to the adjacent soft tissue due to the laceration at the bottom of the vestibule, resulting in loss of substance and exposure of the bone structure. In addition, the fracture exhibited a block fracture pattern, reducing the blood supply in the region. In this context, ozone is recognized as a multifunctional substance, standing out not only for its antibacterial, anti-inflammatory, and analgesic properties but also for playing a stimulating role in vascularization. This favors the proliferation of fibroblasts and bone cells, thus contributing to the tissue healing process [18].
Bilge et al. conducted an investigation using experimental models in mice to evaluate the effects of applying 2ml of ozone in gaseous form, at a concentration of 30μg, over three weeks. The results showed improvements in bone healing in mice induced with osteomyelitis caused by strains of Staphylococcus aureus when compared to groups without therapeutic intervention. It was concluded that ozone has a promising effect as an adjunct therapy for osteomyelitis, increasing antioxidant mechanisms and reducing oxidative stress [19].
In a similar context, other studies have looked at the efficacy of therapy using a single application of ozonated saline solution, with concentrations ranging from 0.5μg/ml to 8μg/ml, on human keratinocyte cells and mouse fibroblast cell lines. These cells were exposed to the bacterial action of S. aureus and Candida albicans fungi, and compared to a control group using 0.2% chlorhexidine, as well as the combination of this with 8μg/ml ozone. Analyses carried out at intervals of 24 and 48 hours revealed subtle effects of ozone therapy on microorganisms, with an increasing relationship with the concentration of the compound. However, the combination of ozone (8μg/ml) and chlorhexidine (0.2%) showed superior antibacterial and antifungal activity when compared to ozone alone (Borges et al. 2017).
In addition, the research included an evaluation of the cytotoxic action on fibroblasts, highlighting the non-toxicity of ozone. These results confirmed ozone’s potential in wound healing since it induced fibroblast migration in the cells analyzed (Borges et al. 2017). Despite the time limitation in the applications and analyses, suggesting a subtle effect, the findings corroborate the present research, highlighting the importance of the therapeutic applied not only in bacterial reduction but also in improving the healing of both bone and epithelial tissue. Additional studies investigated the daily application of 0.1ml of ozonized oil to wounds induced in mice over three, seven, and 11 days.
During the three days, no statistically significant differences were observed between the ozonated group and the control group, showing relevance only on the 7th day, when the treated group showed greater efficacy in revitalizing the wound (Kim et al. 2009). This result can be attributed to ozone’s ability to express growth factors in an enhanced manner, including Platelet-Derived Growth Factor (PDGF), Transforming Growth Factor Beta (TGF-β), and Vascular Endothelial Growth Factor (VEGF). These factors are essential in proliferation and migration of cells crucial in tissue repair, angiogenesis, and control of inflammatory intensity [20,21].
In the context of this clinical case, the daily application of ozonized oil integrated into the protocol combined with two other forms of administration (gas and water) showed an accelerated effect on healing compared to the conventional techniques used on the patient. This accelerated effect can be attributed to the immunological action triggered by ozone in the body, stimulating fibroblasts without inducing cytotoxicity, promoting angiogenesis, and controlling inflammatory responses to favor epithelial and bone regeneration. In the present study, the application of ozone resulted in wound healing, improving tissue regeneration, and controlling the chronic infectious process.
Conclusion
It can be concluded that osteomyelitis represents a complex and challenging condition in terms of its treatment. The primary objective is to eradicate the infectious process, given the concern about the excessive and indiscriminate use of antibiotics, which can favor the emergence of resistant bacterial strains, further complicating the management of this infection. In this challenging context, the application of ozone has proved effective, emerging as a promising alternative for controlling infection and promoting healing. This approach not only offers an effective therapeutic response but can also contribute to mitigating the challenges associated with bacterial resistance, thus reinforcing its innovative potential in the treatment of osteomyelitis.
References
- Boffano P, Roccia F, Gallesio C, Berrone S (2013) Pathological Mandibular Fractures: A Review of the Literature of the Last Two Decades. Dent Traumatol 29(3): 185-196.
- Wagner KW, Otten JE, Schoen R, Schmelzeisen R (2005) Pathological Mandibular Fractures Following Third Molar Removal. Int J Oral Maxillofac Surg 34(7): 722-726.
- Pan A, Lorenzotti S, Zoncada A (2008) Registered and Investigational Drugs for the Treatment of Methicillin-Resistant Staphylococcus aureus Infection. Recent Pat Antiinfect Drug Discov 3(1): 10-33.
- El Meligy OA, Elemam NM, Talaat IM (2023) Ozone Therapy in Medicine and Dentistry: A Review of the Literature. J Dent 11(8): 187.
- Bocci V, Zanardi I, Travagli V (2011) Oxygen/Ozone as a Medical Gas Mixture. A Critical Evaluation of the Various Methods Clarifies the Positive and Negative Aspects. Medical Gas Research 1(1): 6.
- Rangel K, Cabral FO, Lechuga GC, Carvalho JPRS, Villas-Bôas MHS, et al. (2021) Detrimental Effect of Ozone on Pathogenic Bacteria. Microorganisms 10(1): 40.
- Hidalgo-Tallón FJ, Torres-Morera LM, Baeza-Noci J, Carrillo-Izquierdo MD, Pinto-Bonilla R (2022) Updated Review on Ozone Therapy in Pain Medicine. Front Physiol 13: 840623.
- Chen H, Yu B, Lu C, Lin Q (2013) The Effect of Intra-Articular Injection of Different Concentrations of Ozone on the Level of TNF-α, TNF-R1, and TNF-R2 in Rats with Rheumatoid Arthritis. Rheumatol Int 33(5): 1223-1227.
- Serra MEG, Baeza-Noci J, Mendes Abdala CV, Luvisotto MM, Bertol CD, et al. (2023) The Role of Ozone Treatment as Integrative Medicine. An Evidence and Gap Map. Front Public Health 16(10): 1112296.
- Rochford ETJ, Sabaté Brescó M, Zeiter S, Kluge K, Poulsson A, et al. (2016) Monitoring immune responses in a mouse model of fracture fixation with and without Staphylococcus aureus osteomyelitis. Bone 83: 82-92.
- Willenegger H, Roth B (1986) Treatment tactics and late results in early infection following osteosynthesis. Trauma surgery 12(5): 241-246.
- Föger-Samwald U, Dovjak P, Azizi-Semrad U, Kerschan-Schindl K, Pietschmann P (2020) Osteoporosis: Pathophysiology and therapeutic options. EXCLI Journal 19: 1017-1037.
- Singh S, Sarma DK, Verma V, Nagpal R, Kumar M (2023) From Cells to Environment: Exploring the Interplay between Factors Shaping Bone Health and Disease. Medicine 59(9): 1546.
- Al Bari AA, Al Mamun A (2020) Current Advances in the Regulation of Bone Homeostasis. FASEB Bio Advances 2(11): 668-679.
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nat Med 25(12): 1822-1832.
- Epsley S, Tadros S, Farid A, Kargilis D, Mehta S, et al. (2021) The Effect of Inflammation on Bone. Front Physiol 11: 511799.
- Saleh R, Taha RZ, Sasidharan NV, Toor SM, Alajez NM, et al. (2021) Transcriptomic Profiling of Circulating HLA-DR - Myeloid Cells, Compared with HLA-DR + Myeloid Antigen-presenting Cells. Immunol Invest 50(8): 952-963.
- Fitzpatrick E, Holland OJ, Vanderlelie JJ (2018) Ozone Therapy for the Treatment of Chronic Wounds: A Systematic Review. Int Wound J 15(4): 633-644.
- Bilge A, Öztürk Ö Adali Y, Üstebay S (2018) Could Ozone Treatment be a Promising Alternative for Osteomyelitis? An Experimental Study. Acta Ortop B 26(1): 67-71.
- Travagli V, Zanardi I, Bocci V (2009) Topical Applications of Ozone and Ozonated Oils as Anti-Infective Agents: An Insight into the Patent Claims. Recent Pat Antiinfect Drug Discov 4(2): 130-142.
- Kirkwood KL, Zhang L, Thiyagarajan R, Seldeen KL, Troen BR (2018) Myeloid-Derived Suppressor Cells at the Intersection of Inflammaging and Bone Fragility. Immunol Invest 47(8): 844-854.





























