Summary of Recent Classification of Salivary Gland Lesions: A Review
Vinit Patil1, Abhijeet Sande2*, Prasad Karande3 and Ashwini Rani SR4
1Consulting oral pathologist, India
2Department of OMDR, DYPDS, India
3Professor and head, department of oral pathology & Microbiology, DYPDS, India
4Assistant professor, department of OMDR, India
Submission: February 25, 2023; Published: March 17, 2023
*Corresponding author: Dr. Abhijeet Sande, Department of OMDR, DYPDS, Pune, India
How to cite this article: Vinit Patil, Abhijeet Sande, Prasad Karande and Ashwini Rani SR. Summary of Recent Classification of Salivary Gland Lesions: A Review. Glob J Oto, 2023; 25 (5): 556173. DOI: 10.19080/GJO.2023.25.556173
Abstract
The most recent WHO classification of salivary gland tumours aimed to simplify the categorization, although the pathologist must still deal with more than 30 tumours. These include the addition of two new entities, secretory carcinoma along with sclerosing polycystic adenosis, as well as some name revisions. The most contentious changes were removing “low grade” from polymorphous low-grade adenocarcinoma and incorporating intraductal carcinoma as a unifying element. Changes in vocabulary or categorization that are more subtle may also affect the way a diagnostic report is produced. Despite breakthroughs in immunohistochemistry in addition to molecular pathology, the WHO continues to rely mostly on histomorphology for classification. However, physical similarities can make diagnosis challenging without auxiliary procedures. In this study, we detail the revised and new additions to the most recent WHO classification, highlight specific areas of diagnostic difficulty, suggesting potentially effective diagnostic antibodies.
Keywords: Classification; Diagnostic challenges; Immunohistochemistry; Salivary gland tumours; WHO classification
Abbreviations: MEC: Mucoepidermoid Carcinomas; WHO: World Health Organization; AcCC: Acinic Cell Carcinoma; SC: Secretory Carcinoma; AdCC: Adenoid Cystic Carcinoma; ECs: Intermediate Cells
Introduction
These neoplasms of the salivary glands, known collectively as SGT, come in a wide range of shapes and sizes, making accurate diagnosis difficult for the oral pathologist, pathologists, oral surgeons, and oncologists who may encounter them in the course of their work. A malignant tumor of the salivary gland is very rare, making up just around 3% of all head and neck cancers, suggesting a reported incidence of only about 1.2-1.3 instances per 100,000. As a matter of fact, benign tumors make up 80% of all cancers. Most patients are above the age of 40, and there is about an even distribution of men and women among them. However, keep in mind that the male-to-female (M: F) ratio is about 1:1.4 for some of the most prevalent tumors, such as pleomorphic adenoma (PA). Large gland lesions (55%) and tiny gland lesions (50%) are both more likely to be PA than to be malignant, while the great majority of SGT (65%) are benign.
About 20% of SGT occur in the small salivary glands; 70% occur in the parotid gland, 10% in the submandibular gland, plus 1% in the sublingual gland. Whereas malignant tumors in large glands are much more numerous than in minor glands, over half of small gland tumors are cancerous, while only about 20% of big gland tumors are cancerous. Malignant sublingual gland tumors are the exception rather than the rule. Cancers of the parotid gland are made up of 50% PA, 20% Warthin tumors, and 10% mucoepidermoid carcinomas (MEC). In this paper, we address interesting, controversial, and challenging aspects of the most recent WHO categorization of salivary gland tumors and update our earlier evaluations of those modifications. The diagnostic pathologist may benefit greatly from the combination of the most recent fascicle7 of the AFIP and the most recent WHO classification [1-5].
Alterations to the Classification of Salivary Gland Malignancies
A quest to create a universally accepted method for classifying human tumors was initiated in 1952 by the World Health Organization (WHO). Even though Sobin10 has looked into the process, the ultimate goal was to standardize categorization names so that people all around the globe could talk to one other about them. Salivary gland tumors were first categorized by the World Health Organization in 1972. 11 Only 10 primary epithelial SGT were categorized in this first study, and the nomenclature used is almost unrecognizable to modern pathologists with the exception of three terms. There was a dramatic rise in the number of entities included by the time the second edition was issued in 199112. Histomorphological analysis was the backbone of these classifications, which amounted to nothing more than a list of lesions in descending order of prevalence. Surgical oncologists have been particularly vocal in their criticism of the complexity, lack of precision, and inapplicability of such a classification system to current oncological practice.
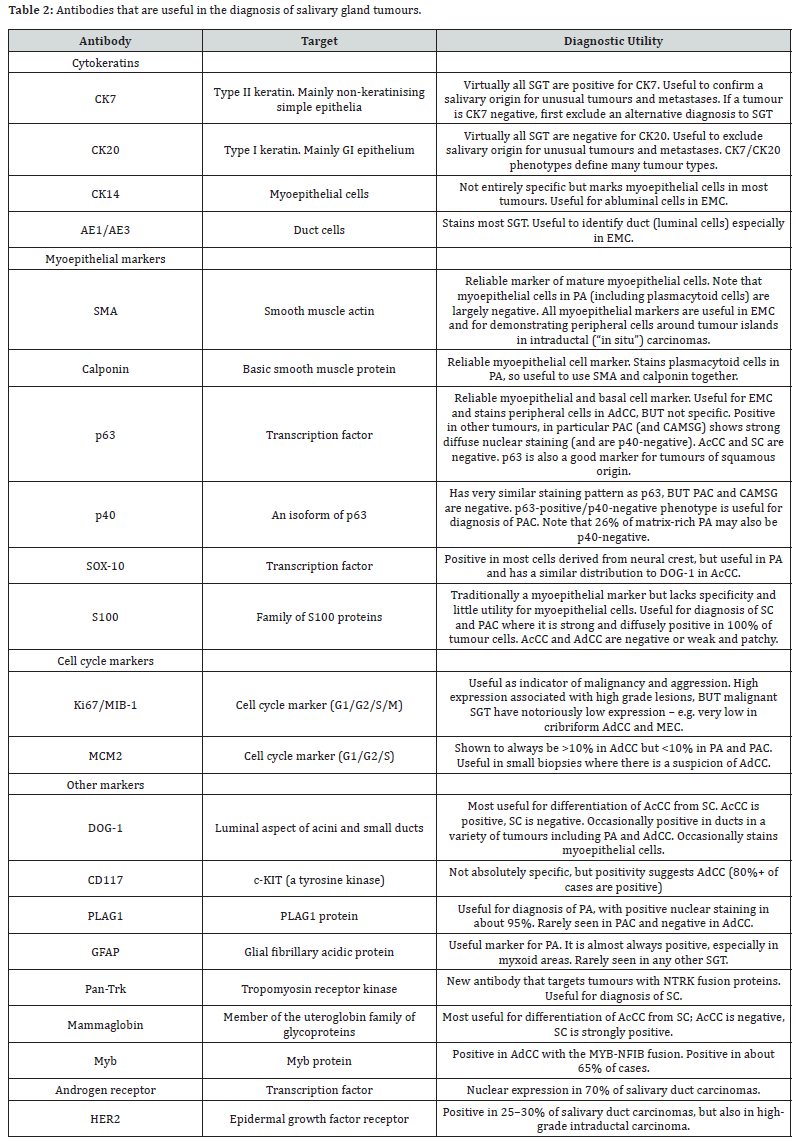
(Table 1) displays the significant alterations to the classification, which are explored in further detail below. The current categorization requires familiarity with everyday diagnostic microscopy and a firm grasp of basic histomorphology. While molecular alterations allow for more precise identification of certain SGT, immunohistochemistry findings are nonetheless presented here. The ones listed in (Table 2) will be discussed in more detail below; nevertheless, it is important to stress that the presence of these ancillary techniques is not necessarily essential for diagnosis or treatment, but may describe a particular organism, when present. Specific gene translocations distinguish two kinds of carcinoma (secretory carcinoma in addition to clear cell carcinoma), however these molecular anomalies are not necessarily present in other tumors (MEC, adenoid cystic carcinoma, cribriform adenocarcinoma of minor salivary glands, PA, and sclerosing polycystic “adenoma”). Because of the precise treatment targets many of these molecular alterations afford, we anticipate that genetic variants will assume a more central role in future classifications as we gain a deeper knowledge of them [5-7].

New Entities
Secretory carcinoma
The absence of PAS-positive intracellular granules, which characterize acinic differentiation, has long been recognized in tumours that otherwise resemble acinic cell carcinoma (AcCC). Within the World Health Organization’s newly streamlined classification system, it is recognized as a kind of secretory carcinoma (SC). Approximately seventy percent of cases of SC occur in the parotid gland, followed by the buccal mucosa, the lips, and the palate. There have only been a few of reported cases where the submandibular or sublingual glands were affected. Some cases have been documented in youngsters however, it affects people of all ages. It seems that men are affected slightly more often than women. While up to 25% of patients have been reported to have nodal metastases, this benign tumour nevertheless has a survival rate higher than 95% [5-9].
Adenosis sclerosing polycystic
There is substantial disagreement over the nature of this lesion, as some sources consider the alterations to be cancerous. Solid evidence for the monoclonal nature of the lesions and, more recently, for the association of sclerosing polycystic adenosis with genetic anomalies in the PI3K pathway as well as PTEN mutations, has been accumulated. This suggests that the lesions in question are really tumours. Curiously, “sclerosing polycystic adenoma” is classified as a synonym, despite the fact that the World Health Organization classifies it as a non-neoplastic epithelial lesion [10,11].
New Titles
Polymorphous adenocarcinoma
In 1984, polymorphous low-grade adenocarcinoma was recognised as a benign tumour that commonly affects the palate. In order to avoid confusion with adenoid cystic carcinoma (AdCC), the term “low grade” was added to the name. We have previously noted that the behaviour of this lesion can be unpredictable, and that some do not behave in a low-grade manner, and we have suggested that the phrase “low grade” be removed from the nomenclature [9-11].
Intraductal carcinoma
Since certain salivary malignancies are thought to be confined inside ductal structures and so can be considered “in situ,” similar to in situ breast ductal tumors, intraductal carcinoma has been proposed as a unique but highly uncommon disease. Numerous types of lesions with an insitu morphology have been reported throughout the years. “By staining with a myoepithelial marker, we can see that the intraductal region of these tumors is surrounded by a layer of intact myoepithelial cells (e.g., calponin, p63, CK14, or smooth muscle actin - see (Table 2).” The new classification from 2017 consolidates all of these illnesses under the umbrella term “intraductal carcinoma,” making diagnosis and treatment easier (Table 1). These lesions are often well-defined and painless growths that affect just the parotid gland. Despite displaying a broad variety of histological traits, they are mostly made up of many cribriform or papillary cystic cavities that resemble ducts [5-8].
Lacking Differentiation Cancer
Poorly differentiated carcinoma is not a newly coined phrase, but rather a novel classification for a collection of highly rare lesions that can only be diagnosed after all other possible primary tumours have been eliminated. This group covers undifferentiated carcinoma as well as neuroendocrine carcinomas with big and small cell sizes.
Clarifications and Modifications
The text of the 2017 classification incorporates a number of subtle revisions, some of which may modify our opinion of a lesion, in addition to the changes in the category of specific tumours, as discussed above and depicted in (Table 1). In particular, we need to think about the reclassification of PA metastasizing and the terminology to be used when reporting ex-PA cancer. Former taxonomies distinguished metastatic PA from malignant tumours of the salivary glands.
Troublesome Diagnostic Regions
Core, impact, and tiny biopsies
H&E-stained sections can be used to diagnose the vast majority of SGT, although enough tissue is needed to evaluate the full range of morphological as well as cytological features. Misdiagnosis at the time of the first biopsy is on the rise because the diagnostic pathologist is typically presented with a small biopsy, and because core and punch biopsies are also encountered frequently. Benign SGT such as PA, basal cell adenoma, canalicular adenoma, Warthin tumour, cystadenoma, and oncocytic lesions can be multifocal and/or capsule-less, further blurring the lines between benign and malignant lesions. However, PA is the only benign SGT that can return, and with to developments in surgical care (particularly extracapsular dissection of parotid tumours), this problem has been largely eliminated. Intravascular PA, whether real or artificial, is rare but thoroughly established, and it does not affect prognosis. Conversely, malignant tumours that are tiny, early stage, or cytologically bland may be well confined, if not encapsulated (thus accounting for the limitations of cytopathology in SGT). To demonstrate the malignant nature of an SGT, histological evidence of an infiltrative margin is the most crucial criterion; nonetheless, caution is required and a full grasp of SGT histomorphology is vital [9-11].
Clear Cell Cancer
Histologically, it reveals a part of a large palate tumour composed of sheets of transparent cells. The differential diagnosis should include clear cell MEC, clear cell odontogenic carcinoma, clear cell MEC, metastasis (especially renal cell cancer), and clear cell minor salivary gland carcinoma. Odontogenic clear cell carcinoma is one of the most prevalent oral cancers, and although it can only develop in the jawbones, it often manifests as a soft tissue growth with or without ulceration. With regards to the major salivary glands, the differential diagnosis largely consists of MEC, metastasis, and the benign possibilities of clear cell oncocytoma and oncocytic hyperplasia, all of which may contain sheets of clear cells as a significant component. Additional tumour types, such as PA and AcCC, in which clear cells are uncommon but not unheard of, could be included to this group.
Histological evidence of epidermoid (squamous) and mucous cells in solid patches that may or may not contain cytologically unremarkable “intermediate cells” (ECs) is diagnostic of MEC. Also helpful are a “tatty” histological appearance, tumorassociated lymphoid infiltration, and the near proximity of tiny mucous salivary glands, all of which are more commonly detected in parotid than intra-oral tumours. Despite the absence of a signature immunohistochemical profile, the vast majority of MEC are positive for the “glandular” cytokeratins (CK) CK7, CK8, CK18, and CK19. In challenging instances and small ambiguous biopsies, the distinctive t (11; 19) (q21; p13) translocation and CRTC1-MAML2 gene fusion can also be a valuable diagnostic, but only if molecular tools are available. Conventional histology methods are preferable over MAML2 alterations for grading and prognosis.
Most cases of clear cell carcinoma have a hyalinized stroma that splits the normally solid sheets of clear cells and an absence of mucous, epidermoid, and/or intermediate cells. In addition to the unique EWSR1 rearrangement, physical differences distinguish MEC from clear cell carcinoma. Since EWSR1 rearrangement is shared by both clear cell carcinoma and odontogenic clear cell carcinoma, telling the two apart may prove especially difficult. However, CK7, CK8, and CK18 expression are sometimes absent in odontogenic carcinomas, while CK19 expression is usually present. Similarly, radiology can be used to trace the intraosseous beginnings of a central odontogenic tumour. Vimentin, PAX-8, and CD10 immunohistochemical positivity, together with a lack of CK7 staining, will help confirm the diagnosis [1,9-11]. The clear cell tumour could be a metastasis from the kidney, in which case the patient’s medical history would be very helpful.
Conclusion
There is a lot of overlap in the physical characteristics of salivary gland tumours, making diagnosis difficult. As the number of tumour types increases, so does the severity of the condition. However, the most recent WHO categorization still includes over thirty entities, despite efforts to simplify the taxonomy. Multiple major name changes have been made, and two new entities have been added to the medical lexicon: secretory carcinoma (formerly known as mammary analogue secretory carcinoma) and sclerosing polycystic adenosis. Due to its unpredictable behaviour, polymorphous low grade adenocarcinoma has been renamed polymorphous adenocarcinoma.
Despite the World Health Organization’s continued emphasis on histomorphological traits as the primary basis for classification, SGT can be difficult to diagnose due to its morphological diversity and overlapping characteristics. Palate biopsies are notoriously difficult for pathologists, as are core biopsies from massive glands. When it comes to the differences between PA, PAC, and AdCC, “pattern-matching” is potentially dangerous, incorrect, and can cause confusion. Even though immunohistochemistry necessitates careful observation and the identification of subtle cytological traits, it might be effective in some situations. These days, there are many different antibodies accessible, and they can be used to help diagnose diseases or distinguish between different kinds of tumours.
References
- PM Speight, AW Barrett (2002) Salivary gland tumours. Oral Dis 8(5) 229-240.
- HB Hellquist, A Skalova (2014) Histopathology of the salivary glands. Springer-Verlag Berlin Pp: 449.
- LDR Thompson, JA Bishop (Eds.) (2017) Head and neck pathology. (3rd edn) Elsevier Pp: 784.
- GL Ellis, PL Auclair (2008) Tumors of the salivary glands. AFIP atlas of tumor pathology Fascicle 9 (4th series), ARP Press, Silver Spring.
- (2017) World Health organization classification of head and neck tumours AK ElNaggar, JKC Chan, JR Grandis, T Takata, P Sootweg (Eds.), Tumours of the salivary glands (In: 4th edn), Lyon IARC press, Pp: 159-202.
- SA Khurrram, AW Barrett, PM Speight (2017) Diagnostic difficulties in lesions of the minor salivary glands Diagnostic Histopathology 23(6): 250-259.
- LH Sobin (1971) The World Health Organization's programme for the histopathological definition and classification of tumours Methods Inf Med 10(2): 120-122.
- AC Thackray, LH Sobin (1972) Histological typing of salivary gland tumours. International histological typing of tumours. No 7 World Health Organization, Geneva.
- G Seifert, LH Sobin (1991) Histological typing of salivary gland tumours. World Health organization international histological classification of tumours (2nd edn.), Springer-Verlag, Berlin.
- L Barnes, JW Eveson, P Reichart, D Sidransky (Eds.) (2005) World Health organization classification of tumours. Pathology and genetics of head and neck tumours, IARC press, Lyon, Pp: 209-281.
- SA Khurram, J Sultan-Khan, N Atkey, PM Speight (2016) Cytogenetic and immunohistochemical characterization of mammary analogue secretory carcinoma of salivary glands Oral Surg Oral Med Oral Pathol Oral Radiol 122(6): 731-742.





























