Transcanal Endoscopic-Assisted Skull Base Resection: Chondromyxoid Fibroma
Wai Tsz Chang1,2*, Cody Chan1, Chan Angela Zaneta3 and Michael CF Tong1,2
1Department of Otorhinolaryngology, The Chinese University of Hong Kong, Hong Kong
2Institute of Human Communicative research, The Chinese University of Hong Kong, Hong Kong
3Department of Anatomical & Cellular Pathology, Prince of Wales Hospital, Hong Kong
Submission:November 10, 2022;Published:November 16, 2022
*Corresponding author: Wai Tsz Chang, FRCS; Department of Otorhinolaryngology, Head and Neck Surgery, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, NT, Hong Kong
How to cite this article: Wai Tsz C, Cody C, Chan A Z, Michael CF T. Transcanal Endoscopic-Assisted Skull Base Resection: Chondromyxoid Fibroma. Glob J Oto, 2022; 25 (4): 556168. DOI: 10.19080/GJO.2022.25.556168
Abstract
Chondromyxoid fibromas (CMFs) are rare, benign cartilaginous bone tumors that are most often found in metaphysis of long bones. This report presents an unusual case of CMF at the lateral skull base that is managed via transcanal endoscopic-assisted techniques. Its feasibility in the resection of lateral skull base tumors, as well as the diagnostic difficulties of CMFs are discussed.
Keywords: Chondromyxoid fibroma; Benign bone tumor; Cartilaginous tumor; Lateral skull base surgery; Transcanal endoscopic techniques
Abbreviations: CMFs: Chondromyxoid Fibromas; MCF: Middle Cranial Fossa; EAC: External Auditory Canal; TMJ: Temporomandibular Joint; MRI: Magnetic Resonance Imaging; PET/CT: Positron Emission Tomography and Computed Tomography; FDG: Fluorodeoxyglucose; MMA: Middle Meningeal Artery; GCT: Giant-Cell Tumors
Introduction
Chondromyxoid fibromas (CMFs) are rare, benign, slow-growing bone neoplasms of cartilage derivation that constitutes less than 0.5% of all bone tumors [1]. Jaffe and Lichtenstein first identified CMFs as distinct entities in 1948 [2], distinguishing them from other malignant pathologies like chondrosarcoma, based on their rarely metastasizing nature. CMFs often present during the second and third decades of life and carries a male predilection [3]. Although CMFs were reported in various anatomical locations, they were most commonly found in metaphysis of long bones (46.9%); cases arising in craniofacial regions are extremely rare and comprise only 5% of all cases [4].
Depending on its location at the skull base, CMF-mediated lesions can result in facial pain, otalgia, hearing loss, tinnitus, diplopia, headaches, restricted motion in the jaw and local swelling that is occasionally accompanied by a palpable mass [5-8]. Histopathological features of CMFs are characterised by well-demarcated lobules composed of stellate or spindle-shaped cells distributed among a myxoid and chondroid background [3]. Given its rarity and striking pathological similarities with other bone tumors, CMFs demonstrated diagnostic difficulties and were often misdiagnosed as malignant lesions. Patients with CMF are typically managed through en bloc resection or curettage. Classic surgical approaches for lateral skull base lesions include the trans petrous route, the trans zygomatic route, the retro sigmoid route and the middle cranial fossa (MCF) approach [9].
Despite being well established for the management of lateral skull base pathologies, these surgical techniques often demand open access with a significant amount of lobular retraction, bone drilling and neurovascular manipulation, which increases post-operative morbidity. With the introduction of transcanal endoscopic techniques, surgeries are now able to utilize the natural corridors of the external auditory canal (EAC) to access pathologies at the level of the petrous bone, petrous apex and internal auditory canal, offering a minimally invasive approach with an improved, panoramic visualization of the surgical field [9]. In this study, we report our experience with skull base resection of CMF that is conducted via transcanal endoscopic-assisted techniques. Its feasibility in the resection of lateral skull base tumors, as well as the diagnostic difficulties of CMFs are further discussed.
Case
A 51-year-old female was presented to the clinic with right side hearing loss for 2 months and mild headaches for years. She was otherwise healthy. On examination, a right ear canal mass was noted. Other neurological examinations were unremarkable. Pure tone audiometry indicated right side maximum conductive hearing loss. Initial biopsy of the mass suggested possible giant cell tumor. Based on the findings, differential diagnoses of chondroid tenosynovial giant cell tumor, chondrosarcoma and osteosarcoma were made. Subsequent contrast-enhanced computed tomography (CT) scan with 3D reconstruction showed a 2.7 x 2.5 x 2.7cm (TD x AP x CC) lobulated, calcified, aggressive lesion centered at the right mandibular fossa with extension to the masticator space and erosion of the anterior right temporal bone, the floor of the right MCF, the anterior wall of the right EAC and the right temporomandibular joint (TMJ) (Figure 1 & 2).
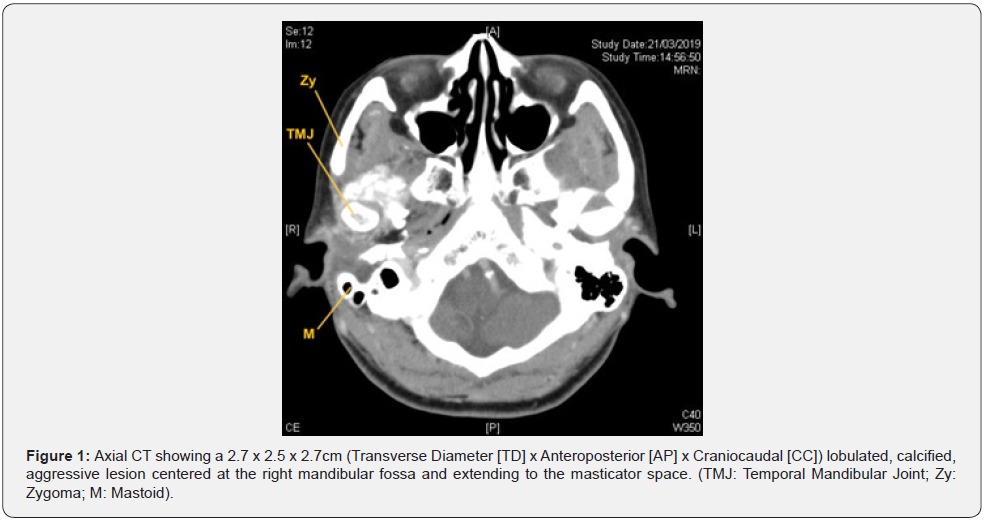
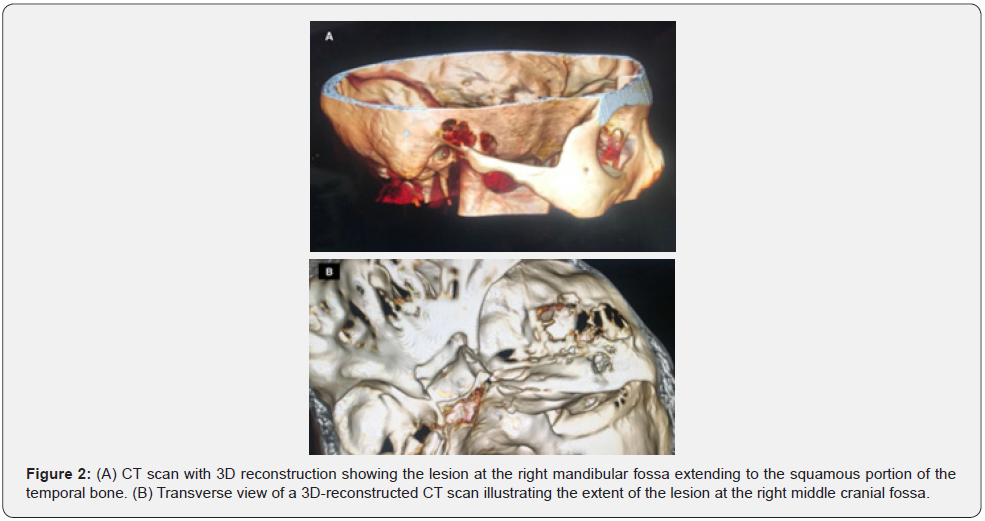
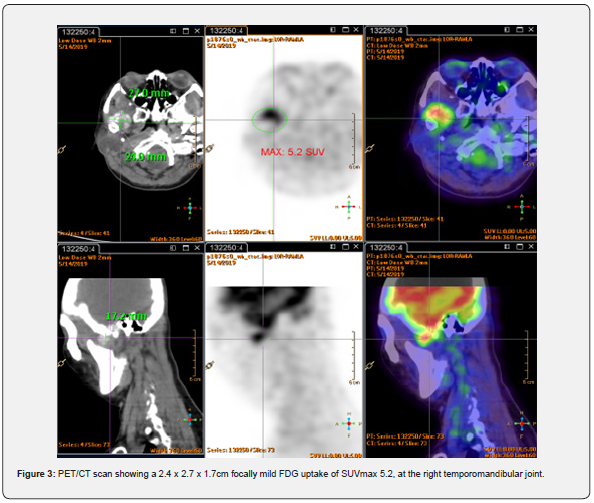
Magnetic resonance imaging (MRI) with contrast revealed a T1 and T2 low signal abnormal mass with minimal peripheral enhancement, interposed between the top of the mandibular condyle and the articular fossa. The mandibular condyle was otherwise preserved, and no dual enhancement was noted. Integrated positron emission tomography and computed tomography (PET/ CT) scan showed the lesion with mild fluorodeoxyglucose (FDG) uptake of maximum standardized uptake value (SUVmax) 5.2 (Figure 3). There was no evidence of FDG-avid distant metastases, but the findings were suspicious of a malignant tumor with local invasion.
Surgical Technique
The patient underwent a general anesthesia tumor resection via a transcanal combined endoscopic-microscopic preauricular approach, in conjunction with a preauricular sinus excision. The incision was continued inferiorly to the ear lobe along the skin crease. A temporalis muscle flap was constructed and elevated showing the squamous portion with tumor exposed superiorly (Figure 4A). Soft tissue dissection continued anteriorly exposing the zygomatic arch and the tumor was reached anteriorly and inferiorly. (Figure 4B). An endoscope was then inserted into the external auditory canal (EAC) for dissection of the tumor involving the superior part of the EAC and the middle ear including the tympanic membrane (Figure 4C). The tumor was involving more than half of the canal so all the EAC skin was removed. Endoscopic bone drilling was performed with curved ultrasonic bone cutting knife (Figure 4D).

Subsequently craniotomy and microscopic atticotomy, to reach the dura mater of the MCF and the posterior border of the tumor respectively. Intraoperatively, the tumor was found posteriorly adhering to the tegmen tympani of middle cranial fossa, involving the EAC and tympanic membrane, anteriorly protruding to the foramen spinosum and foramen ovale, with involvement of the pterygoid plate, dura of the middle cranial fossa, medial and lateral pterygoid (Figure 4E and 4F). The mandibular nerve (V3) and middle meningeal artery (MMA) were also found embedded in the tumor. The MMA was cauterized and cut, and “piecemeal” resection was applied to remove the tumor and preserving the V3. Upon complete ablation of the tumor, blind sac closure was performed. The patient was discharged 2 days after the surgery without any immediate surgical complications.
Pathological Findings
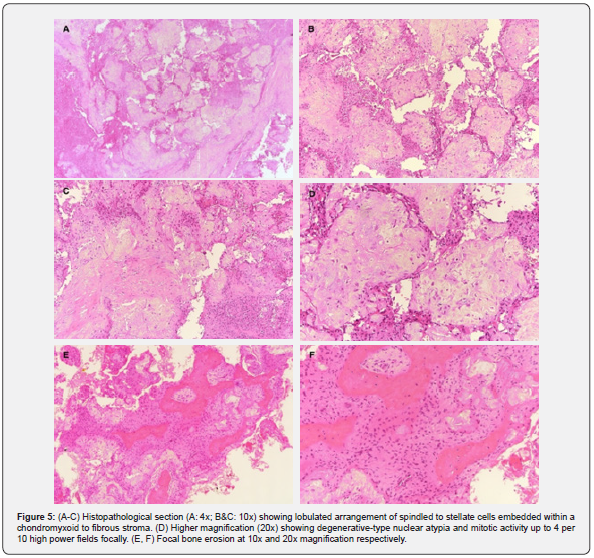
On microscopic examination, the tumor showed a lobulated arrangement of spindled to stellate cells embedded within a chondromyxoid to fibrous stroma. Scattered multinucleated osteoclast- type giant cells were noted. The tumor cells showed degenerative- type nuclear atypia and mitotic activity up to 4 per 10 high power fields focally. No atypical mitotic figures were seen. No sarcomatoid change or necrosis was identified. Focal bone erosion was present (Figure 5). Immunohistochemistry showed focal weak positive staining for S100, and negative staining for SATB2, CD34, IDH-1, H3.3 G34W, CDK4, MDM2 and p53. The case was reviewed in a multidisciplinary meeting and a final diagnosis of chondromyxoid fibroma was established.
Post Operative Course
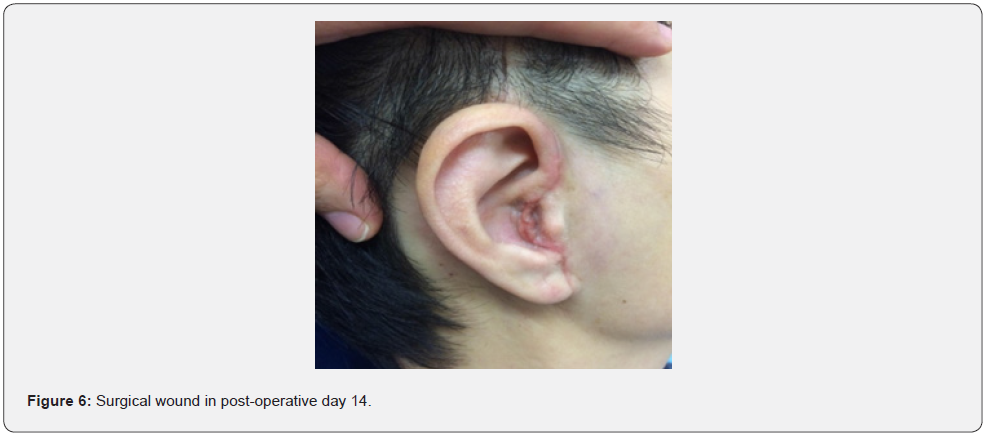
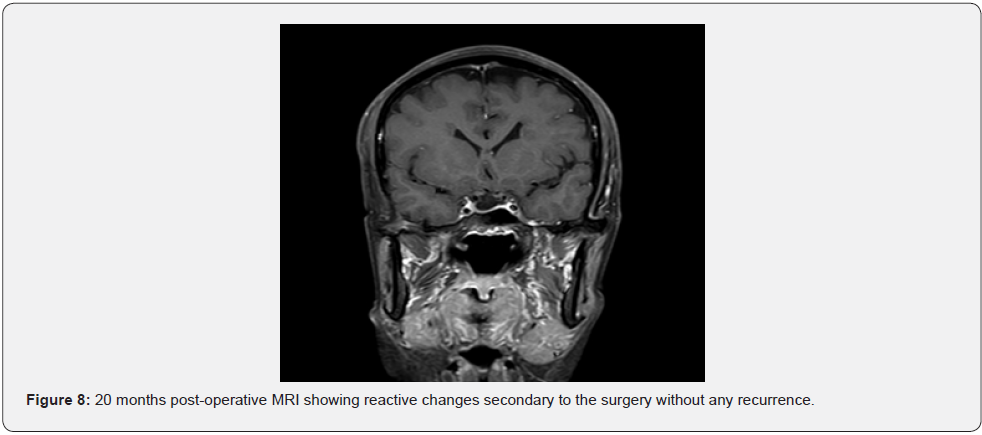
14 days post-operative follow-up reveals minimal scarring over the incision site without any peri-auricular numbness (Figure 6). The patient retained normal function of the facial nerve and mandibular branch of trigermal nerve (V3) with static right side pre-operative hearing loss. We have offered her a bone conductive device for hearing rehabilitation, but the patient declined for normal hearing on left side and no functional deficit. No recurrence was shown in an MRI performed 20 months after the surgery (Figure 7 & 8).

Discussion
Chondromyxoid fibroma is a benign cartilaginous tumor of rare occurrence and is extremely uncommon to arise in craniofacial regions. This report presents a case of CMF that poses considerable diagnostic challenges owing to its rarity, atypical location, inconclusive radiological findings and non-specific preoperative histological features that overlap with malignant lesions. Differential diagnoses of CMFs include aneurysmal bone cysts, giant-cell tumors (GCT), chondrosarcoma, chondroblastoma, enchondroma, non-ossifying fibroma and chordoma [10,11]. Rarely are malignant conversion presented in CMFs though they have been sporadically reported in past literature [4]. In fact, imaging studies of cranial CMFs often reveal aggressive bone destruction, which may confuse cranial CMFs with its malignant mimics [12].
Similar diagnostic pitfalls were presented in our case, where both MRI and CT scans failed to exclude a malignant lesion. Given their inconclusive findings, PET/CT was adjunctly performed to scout for signs of regional or distant metastasis; yet the results were neither helpful in determining the nature of the lesion nor confirming the diagnosis. Pathologically, CMFs pose diagnostic challenges within the craniofacial skeleton as they frequently show bone erosion due to limited room for expansion in this area. In addition, these tumors are often curetted, particularly in this location, which can make it difficult to assess for the characteristic lobulated growth pattern with zones of hypercellularity at the periphery [4]. Potentially worrisome features include focal prominent nuclear atypia and bone infiltration. However, as previously mentioned, CMFs frequently show bone cortical thinning or erosion in craniofacial locations. Therefore, the presence of this feature alone is not sufficient to suggest malignancy.
The overall lack of nuclear atypia and mature cartilaginous elements are key features to distinguish CMFs from well-differentiated chondrosarcoma [12]. Management options of CMF mainly includes in bloc resection and curettage, with the former being the preferred first-line treatment. Overall, there is a 33% recurrence rate for craniofacial CMFs [12], with a surge to 80% when treated with curettage alone (although this is not specific to cranial CMFs) [13,14], in their review of 67 skull base CMFs, reported 1 case of recurrence following gross total resection, but 8 associated with subtotal resection. Due to the relatively high rates of occurrence after curettage, complete resection is often recommended. Other reasons to decide for radical excision include reducing risks of malignant transformation, post-operative cerebrospinal fluid leakage and meningeal infections [6,15]. However, it should be noted that a wide resection performed in craniofacial regions may result in severe functional and cosmetic consequence [15].
Indication for whole-block resection as a result, should be based on tumor location, operative risks, patient preference and functional needs at the site [6,16]. Radiation therapy may be offered for cases involving surgically inaccessible sites. Otherwise, they should be contraindicated considering their risk of inducing malignant conversion and subsequent recurrence [14]. Though certain authors have argued that postoperative radiotherapy is effective in preventing local recurrence [11], it remains controversial whether it is beneficial to the treatment outcome [14]. The choice of surgical approach hugely depends on the location and extent of the tumor. Typical in the context of lateral skull base lesions are the transpetrous routes, the transzygomatic route, the retrosigmoid route and the MCF approach; all of which are open and microscopic-dependent [9].
Our case involves an antero-superiorly located tumor extending to the skull base of MCF. This extent of lesion usually requires a MCF combined transzygomatic approach, which allow access to the entire middle cranial fossa and permits early identification and preservation of critical anatomical structures, including the facial nerve, middle meningeal artery and the internal carotid artery. Hearing can also be well-conserved. This approach, however, involves a large external incision continuing from the frontotemporal hairline to the postauricular sulcus, as well as excessive bone drilling, which undermines post-operative recovery. Temporal lobe retraction during the process may also lead to immediate mechanical damage of the brain, posing risk of epilepsy, brain hematoma or brain herniation [17-19]. Minimally invasive transcanal approach addresses the above issues by utilizing the EAC as a natural surgical corridor to access lesions at the lateral skull base, avoiding meningeal manipulation while minimizing external wounding and bone removal, which significantly reduce operative time, morbidity and improve cosmesis [9].
Further, the use of microscope in conventional approaches can only provide a straight observing view and often requires sacrificing additional soft tissues or bone structures to ensure adequate light reaching the surgical field [20]. Endoscope, on the contrary, are well-known for its wider angle of view, enhanced magnification and its ability to visualize previously “hidden areas” using angled scopes. In fact, previous experiences have demonstrated that the entire inner ear and the temporal bone can be accessed in an exclusive or assisted endoscopic manner, thereby allowing their applications to extend beyond middle ear operations to lateral skull base surgeries [9].
Still, main disadvantages of endoscopic approach include the lack of depth perception and the need for a skill-demanding single-hand operation, which represents a true challenge to otologic surgeons [21,22]. The endoscopic combined microscopic approach documented in our case integrates the unique advantages of microscope and endoscope, whereby improving visualization of anatomical sites to facilitate complete resection and preserving two-hands technique should significant bleeding be encountered [23]. Our patient was able to perform a complete resection via the transcanal endoscopic-assisted approach with excellent functional and cosmetic results, and no recurrence throughout 2 years of follow-up.
Conclusion
Our case has demonstrated the feasibility and potential of transcanal combined endoscope-microscopic approach in the resection of an aggressive skull base CMF. Considering the rarity of CMF and its resemblance to other entities, an integration of clinical, radiological and pathological investigations is crucial in its diagnosis.
References
- Unni K, Inwards C, Mayo Foundation for Medical Education Research, & Mayo Graduate School of Medicine (2010) Dahlin's bone tumors: General aspects and data on 10,165 cases (6th ed.). Philadelphia: Wollters Kluwer Health/Lippincott Williams & Wilkins.
- Jaffe HL, Lichtenstein L (1948) Chondromyxoid fibroma of bone; a distinctive benign tumor likely to be mistaken especially for chondrosarcoma. Arch pathol 45(4): 541-551.
- Fletcher C, Bridge J, Hogendoorn P, Bridge J, Hogendoorn P (2013) WHO Classification of Tumors of Soft Tissue and Bone. Lyon: International Agency for Research on Cancer (IARC) (UN).
- Wu CT, Inwards CY, O'Laughlin S, Rock MG, Beabout JW, et al. (1998) Chondromyxoid fibroma of bone: a clinicopathologic review of 278 cases. Hum Pathol 29(5): 438-446.
- Liu T, Yao J, Li X, Qi X, Zhao P, et al. (2020) Chondromyxoid fibroma of the temporal bone: A rare case report. Medicine 99(11): e19487.
- Feuvret L, Noel G, Calugaru V, Terrier P, Habrand JL (2005) Chondromyxoid fibroma of the skull base: differential diagnosis and radiotherapy: two case reports and a review of the literature. Acta Oncol 44(6): 545-553.
- Sharma M, Velho V, Binayake R, Tiwari C (2012) Chondromyxoid fibroma of the temporal bone: A rare entity. J Pediatr Neurosci 7(3): 211-214.
- Santini-Araujo E, Kalil R, Bertoni F, Park Y (2020) Tumors and Tumor-Like Lesions of Bone. Cham: Springer International Publishing AG.
- Marchioni D, Alicandri-Ciufelli M, Rubini A, Presutti L (2015) Endoscopic transcanal corridors to the lateral skull base: Initial experiences. Laryngoscope 125 (Suppl 5): S1-S13.
- Cappelle S, Pans S, Sciot R (2016) Imaging features of chondromyxoid fibroma: report of 15 cases and literature review. Br J Radiol 89(1064): 20160088.
- Keel SB, Bhan AK, Liebsch NJ, Rosenberg AE (1997) Chondromyxoid fibroma of the skull base: a tumor which may be confused with chordoma and chondrosarcoma. A report of three cases and review of the literature. Am J Surg Pathol 21(5): 577-582.
- Meredith DM, Fletcher C, Jo VY (2018) Chondromyxoid Fibroma Arising in Craniofacial Sites: A Clinicopathologic Analysis of 25 Cases. Am J Surg Pathol 42(3): 392-400.
- Gherlinzoni F, Rock M, Picci P (1983) Chondromyxoid fibroma. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am 65(2): 198-204.
- Yaghi NK, DeMonte F (2016) Chondromyxoid Fibroma of the Skull Base and Calvarium: Surgical Management and Literature Review. J Neurol Surg Rep 77(1): e023-e34.
- Baujat B, Attal P, Racy E, Quillard J, Parker F, et al. (2001) Chondromyxoid fibroma of the nasal bone with extension into the frontal and ethmoidal sinuses: report of one case and a review of the literature. Am J otolaryngol 22(2): 150-153.
- Wang Y, Cao Z, Gu Z (2022) Endoscopic Resection of a Rare Chondromyxoid Fibroma from the Nasal Septum. J Craniofac Surg 33(5): e465-e467.
- Roland PS, Marple BF (1998) The middle cranial fossa approach in managing lesions of the temporomandibular joint. Skull Base Surg 8(1): 11-16.
- Chotai S, Kshettry VR, Petrak A, Ammirati M (2015) Lateral transzygomatic middle fossa approach and its extensions: surgical technique and 3D anatomy. Clinical Neurol Neurosurg 130: 33-41.
- Kim MW, Ryu NG, Lim BW, Kim J (2016) Temporal Lobe Retraction Provides Better Surgical Exposure of the Peri-Geniculate Ganglion for Facial Nerve Decompression via Transmastoid Approach. Yonsei Med J 57(6): 1482-1487.
- Koen N, Remenschneider A, Lee DJ, Kozin ED (2021) Georg von Bekesy and Bruce Mer: Early Pioneers of Endoscopic Ear Surgery. Otolaryngol Head Neck Surg 164(5): 1065-1067.
- Isaacson B, Killeen DE, Bianconi L, Marchioni D (2021) Endoscopic Assisted Lateral Skull Base Surgery. Otolaryngol Clin North Am 54(1): 163-173.
- Pradhan S, Chappity P, Nayak A, Pradhan P, Parida PK (2021) Exclusive endoscopic transcanal approach to lateral skull base lesions: Institutional experience of 3 cases. J Otol 16(1): 55-60.
- Patel EJ, Deep NL, Liu CZ, Jethanamest D (2022) Endoscopic Transcanal and Transmastoid Laser-Assisted Resection of Middle Ear Capillary Hemangioma. Otol Neurotol 43(1): 90-93.





























