Laryngomalacia and OSAS in Young Children: A Case Series
Marcella Gallucci1*, Domenico Saggese2, Emanuela di Palmo1, Ottavio Piccin2, Egidio Candela3*, Angela Miniaci1 and Andrea Pession1*
1Pediatric Unit, Department of Medical and Surgical Sciences, S Orsola University Hospital, Italy
2Department of Otolaryngology Head and Neck Surgery, S Orsola University Hospital, Italy
3Specialty School of Pediatrics, Alma Mater Studiorum, University of Bologna, Italy
Submission:October 06, 2022;Published:October 14, 2022
*Corresponding author: Andrea Pession, Marcella Gallucci and Egidio Candela, Pediatric Unit, Department of Medical and Surgical Sciences, S Orsola University Hospital, Via Massarenti 11, 40138 Bologna, Italy
How to cite this article: Marcella G, Domenico S, Emanuela di P, Ottavio P, Egidio C, et al. Laryngomalacia and OSAS in Young Children: Glob J Oto, 2022; 25 (3): 556165. DOI: 10.19080/GJO.2022.25.556165
Abstract
During childhood, the Obstructive Sleep Apnea Syndrome (OSAS) represent a very common disease with a prevalence that range from 1% to 4%. Laryngomalacia is considered an uncommon cause of OSAS even though it can play a significant role in younger children especially during the first year of life. Congenital laryngomalacia is a common cause of stridor among infants, it is defined as collapse of supraglottic structures during inspiration and in more serious cases it may results in failure to thrive, feeding difficult, aspiration and obstructive disease during sleep. Both the “drug induced sleep endoscopy” (DISE) and flexible fiberoptic laryngoscopy are useful tools to identify respectively the level of upper airway obstruction and the severity and type of laryngomalacia. We present a brief case series comprising 5 children with laryngomalacia where we found a high prevalence of OSAS. All children underwent a sleep study (nocturnal Polysomnography or nocturnal pulse oximetry) and subsequently a fiberoptic laryngoscopy. All showed OSAS associated with laryngomalacia, and they were treated with CPAP therapy with objective improvement of apneas/desaturations number as documented by polysomnographic or pulse oximeter monitoring.
Keywords: Laryngomalacia; OSAS; Flexible laryngoscopy; DISE; CPAP
Abbreviations: LM: Laryngomalacia; OSAS: Obstructive Sleep Apnea Syndrome; GER: Gastroesophageal Reflux; DICE: Drug Induced Sleep Endoscopy; OAHI: Obstructive Apnea-Hypopnea; CPAP: Continuous Positive Airway Pressure
Introduction
Few studies have investigated the larynx as the origin site of sleep apnea. The incidence of OSAS in infants with laryngomalacia (LM) is unknown, although obstructive apnea can be a sign of severe laryngomalacia [1,2]. The real etiology of the congenital laryngomalacia (LM) is unknown. Among infants, immature laryngeal cartilages or abnormal sensorimotor integration may cause inspiratory collapse of supraglottic structures [3]. In addition, gastroesophageal reflux (GER) is frequently associated with this disorder and several authors suggested that the mucosal inflammation due to GER disease increase the severity of symptoms [4]. Moreover, LM can be associated with neuromuscular diseases both congenital and acquired with a prevalence that widely range from 8% to 50% [5].
Stridor is the main finding of congenital laryngomalacia that usually appears after first weeks of life and may increase during feeding, in supine position or restlessness. Some child with severe disease (approximately 10% of cases) may experience more serious symptoms such as failure to thrive, feeding difficulties, respiratory distress, aspiration and obstructive disease during sleep. Nevertheless, most symptoms generally resolve within 2 years of life [1]. The gold standard to confirm the diagnosis of LM and exclude other causes of supraglottic obstruction is the flexible fiberoptic laryngoscopy that is generally performed under general anesthesia during childhood. To ensure the maintenance of a spontaneous breathing, inhalation anesthetics should be used [6,7].
Several classifications of congenital laryngomalacia have been proposed, often based on anatomic (or static) abnormalities or dynamic changes observed during laryngoscopy, even thought to date no codified classification system is accepted. Static findings include omega-shaped epiglottis, acute angle of epiglottis (Holinger et al) and short aryepliglottic folds but these anomalies do not take into account the dynamic changes during spontaneous breathing, therefore, these findings are not optimal for a practice classification system [8]. The most commonly used classification, proposed by Olney and coll. includes three of the following groups: type 1- prolapse of the mucosa overlying the arytenoid cartilages; type 2- foreshortened aryepiglottic folds; type 3- posterior displacement of the epiglottis [9]. Sometimes different combinations of the various types can be observed and the disease can be associated with others lesions such as tracheomalacia, bronchomalacia, subglottic stenosis, and vocal cord paralysis [10].
A recent classification system (Groningen classification) identifies three types, all based on dynamic laryngeal changed:
a) type 1: inward, antero-caudal collapse of arytenoids cartilages;
b) type 2: medial displacement of aryepiglottic fold during inspiration;
c) type 3: postero-caudal displacement of epiglottis against the posterior pharyngeal wall.
This classification provides more didactic categorization of laryngomalacia and may be more practical to guide the best therapeutic approach [11]. Even in older children, laryngomalacia may contribute to OSAS [12]. Isaacson et al. [13] in 1997 used the term “state-dependent laryngomalacia (SDL)” to indicate a child who present normal breathing while awake but stridor and sleep respiratory disorders during sleep. It is unclear whether laryngomalacia that appears later in life is a continuum of a congenital disease or rather a condition that independently develops due to neuromuscular abnormalities. Nevertheless, laryngomalacia even in older children can cause obstructive sleep apnea syndrome due to redundant mucosa prolapsing over the arytenoids upon inspiration. Among these children, the stridor is present less frequently [14]. Among children < 2 years with OSAS, the pattern of upper airway obstruction is poorly known since data are lacking and often derived by retrospective studies. The diagnosis can be achieved by drug induced sleep endoscopy (DISE) that consist of the upper airway inspection during induced sleep through light general anesthesia.
DISE provides a global evaluation of sites of obstruction during sedation and therefore can identify the level and the severity of collapse [15,16]. Therefore, it may be useful to plan the best surgical approach for children with persistent OSA, even though a standardized method of interpretation that ensure an acceptable agreement among operators is lacking. DISE can also be indicated also for children who have persistent OSA after adenotonsillectomy, those with OSA without tonsillar hypertrophy and in older children who laryngomalacia is suspected [17]. Boudewyns et al. [15] in their retrospective study involving 28 two-year-old children, found a picture of collapse of epiglottis in 6 patients and laryngomalacia in other 4 subjects. All the children had severe OSAS. Among these children, DISE was useful in surgical decision making in order to achieve a targeted treatment based on the site of obstruction.
Cases Presentation
We presented a case series of 5 children with OSAS associated with laryngomalacia in order to emphasize the impact of this condition in the occurrence of obstructive sleep-disordered in small children. All the parents gave an informed consent on data publication. All the children underwent a sleep Polysomnography with EEG channels (Embletta PG MPR/ST+Proxy) and a nocturnal pulse oximetry. Subsequently every children underwent fiberoptic laryngoscopy under slight anesthesia during the same hospitalization. We categorized the laryngomalacia type according to GRONINGEN classification system [11]. We also decided to perform an esophageal X-ray with water-soluble contrast to assess the concomitant presence of aspiration and/or gastroesophageal reflux even thought this technique has a poor sensitivity and specificity for GERD.
This diagnostic tool was used mainly to exclude other disorders such as aspiration, swallowing and motility disorders that can be correlated with apnea among infants [18]. The diagnosis of OSAS was defined by polysomnography findings of OAHI >1 (obstructive apnea-hypopnea index: obstructive and mixed apneas and hypopneas for hour of total sleep time): mild OSAS for OAHI 1-3, moderate for OAHI >5<10, severe OSAS for OAHI >10) [19]. Since physiologic polysomnographic parameters widely vary with age, we used age-appropriate norms according to Chan et al. [20,21]. The patients’ detailed clinical data are presented below (Table 1).
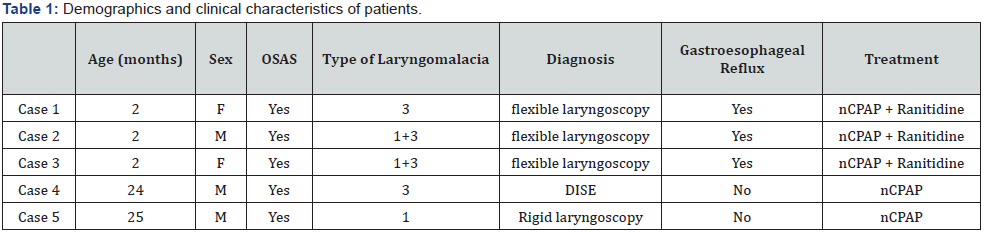
a) Case-1
The first case is a 2-month-old girl with cleft lip who presented with stridor, since the first day of life parents referred nocturnal apneas. The nocturnal polysomnography (PSG) showed a picture of severe OSAS with AHI of 54.1/h (OAHI 37.5). The child also underwent an esophageal X-ray with water-soluble contrast agent that detected multiple episodes of gastroesophageal reflux. For this reason an anti-acid therapy with ranitidine was started. The flexible laryngoscopy performed under sedation revealed a posterior laryngomalacia (type 3 according with Groningen classification). Subsequently, treatment with nasal Continuous Positive Airway Pressure (CPAP) was started at initial pressure of 4cm H2O and gradually increased up to 5cmH2O. The polysomnography performed during nCPAP showed a significant improvement of the clinical picture (AHI 3,8/h). This treatment was well tolerated by the child until complete weaning at the age of about one year old.
b) Case-2
A 2 months-old baby boy was admitted to our center due to biphasic stridor worsening when restlessness.
The child underwent esophageal X-ray that showed multiple episodes of gastroesophageal reflux therefore treatment with ranitidine was started. The nocturnal PSG revealed the presence of both obstructive and central apneas with OAHI of 7.8/h. The examination with flexible laryngoscopy confirmed the suspicion of moderate global laryngomalacia without other airway abnormalities (type 1 + type 3: inward collapse of arytenoids cartilages + posterocaudal displacement of epiglottis against the posterior pharyngeal wall). In this case a treatment with CPAP was also started at the pressure of 4cmH2O. This approach was well tolerated by the child allowing an improvement of apneas number (AHI 3.1/h) as documented by the subsequent polysomnography. NCPAP was interrupted at 10 months due to resolution of the stridor and apneas.
c) Case-3
Another 2 months-old baby girl was admitted to our medical center due to finding of persistent stridor both at rest and in restlessness. The baby underwent nocturnal PSG that detected a picture of severe OSAS (OAHI 15/h). The esophageal X-ray with barium showed repeated reflux episodes with slow esophageal clearance. The flexible laryngoscopy under slight anesthesia revealed a posterior laryngomalacia of moderate degree. The fiberoptic examination also highlighted a slightly rear epiglottis due to the brevity of the aryepiglottic cartilages (type 1 + 3). Even in this case, we decided to start treatment with nasal CPAP at the pressure of 4 cmH2O with rapid improvement of average night saturation and number of obstructive apneas (residual AHI 4/h). Nevertheless, this therapy was discontinued after a few weeks due to poor family compliance.
d) Case-4
2 years-old child male of African ethnicity came to our attention for symptoms of habitual snoring, oral breathing and sleep apnea since the first month of life. The child also showed failure to thrive, daytime hyperactivity and frequent episodes of upper airway infections. The clinical examination showed persistent oral breathing with nasal obstruction, narrow palate, 2° non-occlusive degree tonsils. The PSG revealed a diagnosis of severe OSAS with OAHI of 10,8/h and Oxyhemoglobin Desaturation Index of 9,7/h. Due to a clear discrepancy between the tonsillar hypertrophy degree and the severity of OSAS, a DISE was performed. The sleep endoscopy showed a picture of adenoidal obstructive hypertrophy with lateral pattern of oropharyngeal collapse and kissing of the palatine tonsils.
Moreover, at hypopharyngeal level there was evidence of complete obstruction due to arytenoids prolapse with posterior laryngomalacia of moderate-severe degree (type 3). In this case, DISE highlight a multilevel collapse that probably justified the picture of severe OSAS. Therefore, we started nCPAP treatment with pressure of 5cm of H2O. The nocturnal oximetry performed during CPAP showed improvement of mean saturation and Oxyhemoglobin Desaturation Index (ODI 2/h). The subsequent polysomnography performed under CPAP after about 30 days, showed a resolution of OSAS (AHI 1.5). At the age of three years, the child underwent adeno-tonsillectomy with subsequent weaning from CPAP therapy.
e) Case-5
The last case concerns a 2,5 months-old child male, admitted to our pediatric center for history of stridor since the first days of life associated with episodes of apnea in both wakefulness and sleep. The nocturnal oximetry showed numerous desaturation events with an Oxyhemoglobin Desaturation Index of 25/h. The PSG showed OSAS of severe degree (OAHI 28,5/h) while the esophageal X-ray founded numerous episodes of inhalation of water-soluble contrast agent in the larynx, without GER. The flexible laryngoscopy showed a retro-position of the tongue resting on the posterior wall of the pharynx due to retrognathia. Rigid endoscopy was subsequently performed and founded omega-shaped epiglottis, short aryepiglottic folds and marked inward collapse of arytenoids cartilages. In this case, we also decided to start nCPAP treatment with pressure of 4cm H2O. Due to the agitation and the small age of the baby we decided not to repeat the polysomnography, but only to perform a nocturnal pulse oximetry under CPAP that showed a substantial improvement in mean saturation and Oxyhemoglobin Desaturation Index (ODI 0.4/h). The child was discharged after a few weeks in good general conditions and continued nocturnal ventilation with CPAP to date.
Result
The mean age of children was 6,5 months (range of 2-24 months). Four children presented an endoscopic finding of congenital laryngomalacia with early onset of stridor and OSAS, 1 child showed severe OSAS associated with laryngomalacia, and adeno-tonsillar hypertrophy assessed by DISE. Three children had RGE documented by esophagus X-ray (Table 1) while one child presented with numerous episodes of aspiration. Three subjects had a polysomnographic diagnosis of severe OSAS while one child showed a moderate degree OSAS. Nocturnal oximetry was performed in each child and was interpreted according with Brouillette criteria and the McGill Oximetry score. All children had a positive oximetry [22,23]. Treatment with nasal-CPAP was started in all cases of moderate-severe OSAS. (5 children).
Among these children, overnight sleep study was repeated during the ventilation trial for CPAP titration starting at the lowest value (4cm H2O) until the highest value of 6 cm H2O. The mean nCPAP pressure, used to abolish upper airway obstruction, was 4.4 ± 0.5cm H2O. The nCPAP therapy was effective in all cases treated except one in which it was interrupted after 1 month due to poor family adherence. In this 2-month-old-girl, according to parent decision, we only maintained an anti-reflux therapy and a close clinical follow-up until now. At the hospital discharge, four children received Ranitidine therapy. All children maintained a close monthly follow up in order to assess the need of mask size’s adjustments or change of the CPAP pressure. At monthly clinical control, all maintained good clinical conditions with proper growth and satisfactory adherence to CPAP therapy.
Improvement of obstructive apneas was initially monitored with monthly home pulse oximetry while PSG was performed before weaning from CPAP (PSG was performed in spontaneous breathing). For the case that CPAP was discontinued after a few weeks due to poor family compliance, we decided to maintain a close clinical follow-up associated with antireflux therapy. Two babies discontinued CPAP treatment due to clinical improvement after about 8 months; one child had interrupted the therapy after adeno-tonsillectomy (about 13 months later) while the last child maintained to date long term nCPAP.
Discussion and Conclusions
OSAS is a very common disease that can cause significant morbidity during childhood. Several evidences suggest that SDB has a deleterious effect on infants, particularly on growth and also on intellectual development [20]. Infants with prolonged apnea may show lethargy, feeding difficulty, growth and developmental delay [24]. OSAS risk factors in infants differ from those of older children. Not only in early life but also in older children, the presence of laryngomalacia can cause respiratory disorders and some severe cases requires some treatment. This finding is confirmed by our case series since all children with laryngomalacia showed OSAS. During the first months of life, gastroesophageal reflux worsens the symptoms of laryngomalacia so an appropriate anti-reflux treatment can improve the clinical picture of LM [4,9]. GER induces mucosal edema that worsens the airway narrowing while LM can create an intrathoracic depression that predisposes to GER in a vicious circle [1,9].
Among the listed cases, 3 children showed gastroesophageal reflux assessed by esophageal X-ray and another child showed some symptoms of reflux (frequent regurgitation and irritability) therefore he was empirically treated with ranitidine with rapid clinical improvement (Table 1). According to Groningen classification system, for our patients the most frequent detected type of congenital laryngomalacia was type 3, following by type 1 (Table 1). The flexible laryngoscopy was performed under sedation but with spontaneous breathing so that the endoscopic findings were not distorted. A study of Sivan et coll. [25] showed a risk of false-negative rate of about 8% for laryngoscopy performed in wake vs general anesthesia. Laryngoscopy in conscious infants may miss cases of sleep-induced laryngomalacia, with obvious difficulties in execution due to agitation’s child therefore in our clinical practice, we routinely perform this examination under topical anesthesia and slight sedation.
In one case, we performed a drug-induced sleep endoscopy due to the older age of the child, referred symptoms and the severity of OSAS without an objective adeno-tonsillar hypertrophy. DISE allowed the detection of the obstruction site showing an adenoidal obstructive hypertrophy with lateral pattern of oropharyngeal collapse and it allowed the diagnosis of posterior laryngomalacia of moderate-severe degree due to arytenoids prolapse. In our opinion, among older children with sleep respiratory symptoms and non-occlusive degree tonsils, DISE may be complementary to laryngoscopy in the diagnostic path. Treatment of OSAS associated with laryngomalacia may include conservative management (such as wait and see approach, antireflux treatment and CPAP ventilation) or surgery (summarized under the term of supraglottoplasty) that can be made urgently or in a second time. Among these cases, we found a high effectiveness of nasal CPAP to treat both OSAS and laryngomalacia symptoms.
The CPAP mechanism of action is due to a pneumatic thrust resulting in increase of the upper airways pressure that overcome the critical transmural pressure of the pharynx and the hypopharynx and prevent the airway collapse ant then the obstructive sleep apnea. The nasal mask must adhere to the baby’s face to prevent air leaks. Evidence by literature founded out that among cases of severe laryngomalacia and vocal cord paralysis with OSA, non-invasive ventilation through nasal CPAP represents an effective and safe alternative to surgery [26]. Moreover, nasal CPAP may be a useful approach in children in which upper airway obstruction is not resolved by adenotonsillectomy [27]. The CPAP titration usually begins at the lowest pressure (4cm H2O) and involves a gradually increasing until obstructive sleep apneas and oxyhemoglobin desaturations were overcome. Monitoring of CPAP assessment is recommended every 6–12 months, since mask size and CPAP pressure are likely to change with child growth [28-30].
Among our cases, the CPAP therapy was discontinued after about 8 months in two case due to improvement of symptoms (both stridor and apnea). In another child, CPAP was a useful therapeutic strategy until he underwent the adeno-tonsillectomy (at the age of 3 years) that allowed a resolution of OSAS. For these reasons, CPAP treatment may be a bridging therapy pending the improvement of symptoms or surgery. We found a high CPAP effectiveness and adherence in infants with OSAS, probably due to small age of subjects and the availability in our center of adequate training and support for caregivers. Only in one case, CPAP was interrupted, in our opinion due to poor parent’s perception of the benefits of treatment.
For these reasons, an adequate training program for parents and also the availability of specialized medical and nursing staff is essential to improve the rate of CPAP success. Actually, little evidence is available regarding the indications for polysomnography and others sleep studies in children with laryngomalacia. Recent guidelines suggest that polysomnography should guide therapeutic approach in infants with laryngomalacia associated with cardiac/ neurological diseases or complex malformation syndrome [1]. Nevertheless, we believe that in all cases assessed for laryngomalacia it is prudent searching for OSAS since the prevalence of SDB among this subgroup may be probably more frequent than expected.
References
- S Ayari, G Aubertin, H Girschig, T Van Den Abbeele, M Mondain (2012) Pathophysiology and diagnostic approach to laryngomalacia in infants. Eur Ann Otorhinolaryngol Head Neck Dis 129(5): 257-263.
- CL Marcus, LJ Brooks, KA Draper, D Gozal, AC Halbower, et al. (2012) Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130(3): e714-e755.
- DM Thompson (2007) Abnormal sensorimotor integrative function of the larynx in congenital laryngomalacia: a new theory of etiology. Laryngoscope 117(6 Pt 2 Suppl 114): 1-33.
- H Bibi, E Khvolis, D Shoseyov, M Ohaly, D Ben Dor, et al. (2001) The prevalence of gastroesophageal reflux in children with tracheomalacia and laryngomalacia. Chest 119(2): 409-413.
- J Carter, R Rahbar, M Brigger, K Chan, A Cheng, et al. (2016) International Pediatric ORL Group (IPOG) laryngomalacia consensus recommendations. Int J Pediatr Otorhinolaryngol 86: 256-261.
- TM Lima, DU Goncalves, LV Goncalves, PA Reis, AB Lana, et al. (2008) Flexible nasolaryngoscopy accuracy in laryngomalacia diagnosis. Braz J Otorhinolaryngol 74(1): 29-32.
- Y Sivan, J Ben-Ari, R Soferman, A DeRowe (2006) Diagnosis of laryngomalacia by fiberoptic endoscopy: awake compared with anesthesia-aided technique. Chest 130(5): 1412-1418.
- LD Holinger, RJ Konior (1989) Surgical management of severe laryngomalacia. Laryngoscope 99(2): 136-142.
- DR Olney, JH Greinwald, RJ Smith, NM Bauman (1999) Laryngomalacia and its treatment. Laryngoscope 109(11): 1770-1775.
- EB Hysinger (2018) Laryngomalacia, Tracheomalacia and Bronchomalacia. Curr Probl Pediatr Adolesc Health Care 48(4): 113-118.
- M van der Heijden, FG Dikkers, GB Halmos (2015) The groningen laryngomalacia classification system based on systematic review and dynamic airway changes. Pediatr Pulmonol 50 (12): 1368-1373.
- GT Richter, MJ Rutter, A de Alarcon, LJ Orvidas, DM Thompson (2008) Late-onset laryngomalcia: a variant of disease. Arch Otolaryngol Head Neck Surg 134(1): 75-80.
- MR Amin, G Isaacson (1997) State-dependent laryngomalacia. Ann Otol Rhinol Laryngol 106(11): 887-890.
- Digoy GP, Burge SD (2014) Laryngomalacia in the older child: clinical presentations and management. Curr Opin Otolaryngol Head Neck Surg 22(6): 501-505.
- P Boudewyns, A Van de Heyning, S Verhulst (2017) Drug-induced sedation endoscopy in children <2 years with obstructive sleep apnea syndrome: upper airway findings and treatment outcomes. Eur Arch Otorhinolaryngol 274(5): 2319-2325.
- SO Ulualp, P Szmuk (2013) Drug-induced sleep endoscopy for upper airway evaluation in children with obstructive sleep apnea. Laryngoscope 123(1): 292-297.
- ML Durr, AK Meyer, EJ Kezirian, KW Rosbe, (2012) Drug-induced sleep endoscopy in persistent pediatric sleep-disordered breathing after adenotonsillectomy. Arch Otolaryngol Head Neck Surg 138(7): 638-643.
- Y Vandenplas, CD Rudolph, C Di Lorenzo, Hassall E, Liptak G, et al. (2009) Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr 49(4): 498-547.
- AG Kaditis, MLA Alvarez, A Boudewyns, F Abel, EI Alexopoulos, et al. (2017) ERS statement on obstructive sleep disordered breathing in 1- to 23-month-old children. Eur Respir J 50(6): 1700985.
- AG Kaditis, MLA Alvarez, A Boudewyns, EI Alexopoulos, R Ersu, et al. (2016) Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J 47(1): 69-94.
- DK Ng, CH Chan (2013) A review of normal values of infant sleep polysomnography. Pediatr Neonatol 54(2): 82-87.
- RT Brouillette, A Morielli, A Leimanis, KA Waters, R Luciano, et al. (2000) Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics 105(2): 405-412.
- GM Nixon, AS Kermack, GM Davis, JJ Manoukian, KA Brown, et al. (2004) Planning adenotonsillectomy in children with obstructive sleep apnea: the role of overnight oximetry. Pediatrics 113(1 Pt 1): e19-25.
- D Gozal, L Kheirandish-Gozal (2007) Neurocognitive and behavioral morbidity in children with sleep disorders, Curr Opin Pulm Med 13(6) 505-509.
- Y Sivan, J Ben-Ari, R Soferman, A DeRowe (2006) Diagnosis of laryngomalacia by fiberoptic endoscopy: awake compared with anesthesia-aided technique. Chest 130(5): 1412-148.
- F Massa, S Gonsalez, A Laverty, C Wallis, R Lane (2002) The use of nasal continuous positive airway pressure to treat obstructive sleep apnoea. Arch Dis Child 87(5): 438-43.
- (1994) Indications and standards for use of nasal continuous positive airway pressure (CPAP) in sleep apnea syndromes. American Thoracic Society. Official statement adopted March 1944. Am J Respir Crit Care Med 150(6 Pt 1): 1738-1745.
- CL Marcus, SL Ward, GB Mallory, CL Rosen, RC Beckerman, DE Weese-Mayer, et al. (1995) Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J Pediatr 127(1): 88-94.
- CL Marcus (1999) Advances in management of sleep apnea syndromes in infants and children. Pediatr Pulmonol Suppl 18: 188-189.
- CA Kushida, A Chediak, RB Berry, LK Brown, D Gozal, et al. (2008) Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med 4(2): 157-171.





























