The Clinical Applications of Image Guidance in Revision Endoscopic Frontal Sinus Surgery
Ali Almomen1 and Muneera AlKhalifa2*
1Consultant Rhinology & Skull base surgery, King Fahad Specialist Hospital, Kingdom of Saudi Arabia
2 Bahrain Defense Force Hospital, Bahrain
Submission:July 01, 2020; Published:July 23, 2020
*Corresponding author:Muneera Al Khalifa, Bahrain Defense Force Hospital, Bahrain
How to cite this article: Ali Almomen, Muneera Al K. The Clinical Applications of Image Guidance in Revision Endoscopic Frontal Sinus Surgery. Glob J Oto, 2020; 23 (1): 556101 DOI: 10.19080/GJO.2020.23.556101
Abstract
Background: Frontal sinus disease is obstinate. With its multifaceted anatomy and neighboring vital structures, frontal sinus disease creates a dreaded encounter to every otolaryngologist. Further complicated are revision endoscopic frontal sinus surgeries beside unrecognizable anatomy, revision endoscopic frontal sinus surgeries present technical challenges. The objective is to highlight the causes of revision endoscopic frontal sinus surgery and illustrate the clinical applications of the image guidance in managing them.
Methods: Retrospective review of 60 patients underwent revision endoscopic sinus surgery with image guidance from 2015 to 2019
Results:the causes of revision out of 60 patients 33% were due to retained uncinate process, residual agger nasi with/without ethmoid disease. Followed by 25% due to extensive mucosal disease with polyps then 24%, 11% and 5% presenting lateralized middle turbinate, unopened suprabullar cell and neo-osteogenesis, respectively. All patients were followed up for 3 years with 91.67% successful patency rate of frontal outflow after revision.
Conclusion: the image guidance in revision endoscopic frontal sinus surgery provides accurate identification of remnant bones or cells that may obscure the outflow. Its further aids in evading recurrence or persistent disease with adequate frontal sinusotomy while protecting vital structures.
Keywords:Frontal sinus; Revision endoscopic sinus surgery; Image guided endoscopic sinus surgery
Introduction
Frontal sinus surgeries throughout the history have been tiresome. Even in the hands of experts frontal sinus surgeries outcomes have been debatable. The available success rate of primary functional endoscopic sinus surgeries varies from 76-98% [1]. None the less almost 25% of operated individuals eventually require revision surgery for unrelenting or recurrent symptoms. Published data by Khalil et al. [2] displayed causes of failure including residual frontal air cells, posterior or anterior ethmoid air cells, residual uncinate process, deviated nasal septum or middle turbinate lateralization [3]. Many of which would contribute to frontal recess blockage where the disease is most probably to reoccur [4]. The purpose of this study is to emphasize the causes of failed primary endoscopic frontal sinus surgeries and illustrate the clinical applications of the image guidance in managing them.
Aim
Study design and patients
The medical records of 47 geriatric patients who underwent tracheostomy at our hospital were retrospectively evaluated in this study. Informed consent was not obtained from patients because of the retrospective study design. The study was approved with a waiver of consent by the Local Ethics Committee (Date: 30.05.2019/ MH 35). Patients below the age of 65 years were excluded from the study. At our hospital, tracheostomies are performed by anesthesia and reanimation specialists and otorhinolaryngologists; ST procedures are performed by the latter. Recently, anesthesia and reanimation specialists have begun performing PT. PT and ST were performed in patients at ICU beds and operating rooms, respectively. No muscle relaxants were used for either procedure.
Patients were sedated with midazolam and propofol, and local anesthesia was applied. Patients who underwent PT and ST were evaluated. Age, sex, indication for ICU admission comorbidity status, APACHE II scores, number of intubated days, intubation time, survival days, operation time, complications during and after the procedure (hemorrhage, subcutaneous emphysema, pneumothorax, hypotension, stoma infection, displacement, mortality, necessity of PT conversion to ST, hypoxia and tracheal stenosis), and the presence of tracheostomy during discharge were recorded. The mean operation time was defined as the time from the skin incision to the insertion of the tracheostomy tube.
Open surgical tracheostomy technique
The patients were placed in the supine position with their neck extended. The neck is to be fully extended by placing a shoulder roll underneath the upper back area and the head is stabilized on a head ring [12]. Under sterile conditions, the surgical site was covered. The cricoid cartilage and sternal notch were determined, and the midpoint of the line joining these two points was marked. Local anesthesia was applied to the surgical field. A 1.5cm incision was made. Blunt dissection in the midline through the linea alba is carried out and the strap muscles are retracted laterally using Langen beck retractors. The thyroid isthmus comes into view and this can have a variable position and size. It is skeletonized and can be divided either using clamps and transfixion sutures or using monopolar or bipolar diathermy.
The anterior tracheal wall is identified and cleaned. It is useful to identify the cricoid cartilage so to be able to assess the entry point for the tracheotomy. Tracheotomy was usually performed on the 3rd or 4th tracheal rings, and the area was marked by bipolar diathermy. A conservative square tracheal window was created as much as possible. The membrane tissue was carefully cut between the rings. After the tracheal window was opened, secretion was thoroughly aspirated. The assistant was asked to pull the endotracheal tube. A tracheostomy cannula with an appropriate diameter was inserted through the tracheal window. The tracheostomy cuff was then inflated, and bilateral chest enlargement was confirmed by ventilating the lung with the cannula [12].
Percutaneous dilation tracheostomy technique
The patient is placed in a supine position. To extend the neck, a towel wrapped under the shoulder is placed so that the trachea can be easily palpated, and the cricoid cartilage is better pulled. Tracheal rings are palpated to identify the stern notch and cricoid cartilage. After the patients were positioned, antisepsis of the neck was achieved, and local anesthesia was applied. With the help of a laryngoscope or bronchoscope, the ET cuff was retracted so that it could be seen between the vocal cords. A skin incision of 1-1.5cm was made at the level of the second-third tracheal rings. The location of the needle was confirmed by entering the trachea with 2-3mL saline-filled syringe from the midline and aspirating the air. A J-tipped guidewire was advanced through the needle and the needle was removed. A guiding catheter was inserted over the guidewire. All dilatations were performed on this combination of guidewire and guiding catheter. Dilatations were started with a 12F dilator and sequential expansion was made up to 36F dilator for the 8 mm tracheostomy cannula. The last tracheostomy cannula with cuff was placed over the combination of guidewire and guiding catheter. The tracheostomy balloon was inflated. Lung was ventilated and bilateral chest enlargement was confirmed [13].
Statistical analyses
To study the clinical applications of image guidance in managing the different causes of revision endoscopic frontal sinus surgeries.
Methods and Materials
Retrospective charts review of 60 patients underwent revision endoscopic frontal sinus surgery with image guidance for chronic recurrent sinusitis with and without polyposis from 2010 to 2019 at a tertiary referral hospital of King Fahad Specialist Hospital Dammam , the eastern province of Saudi Arabia with 4 million population.
Results
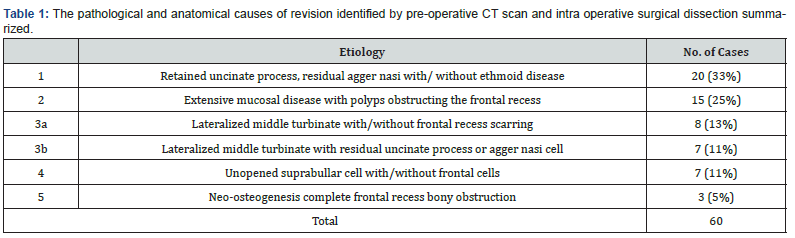
The causes of revision surgery and frontal disease reoccurrence (Table 1) showed variable causes. From the 60 patients reviewed 33% were due to retained uncinate process, residual agger nasi with/without ethmoid disease. Followed by 25% due to extensive mucosal disease with polyps then 24%, 11% and 5% presenting lateralized middle turbinate, unopened suprabullar cell and neo-osteogenesis, respectively (Table 1).
All patients were followed up for the period from 1 to 5 years with 91.67% successful patency rate of frontal outflow after image guided endoscopic revision. Few Illustrative cases demonstrating the value of image guidance in managing different causes of revision endoscopic frontal sinus surgery (Figures 1-9).




Discussion
Frontal sinus surgeries present a challenge due to its unique outflow anatomy. Resting between vital structures as the orbit and base of skull attaining an adequate outflow whilst avoiding complications is a trial. The complexity of frontal recess air cells presents another obstacle in the goal of achieving flow. Whilst taking in consideration these difficulties in a primary case frontal sinusitis in a revision case magnifies the challenge. Interpreting the cause of reoccurrence needs thorough evaluation of the patient’s radiological and clinical evaluation. A CT scan should be performed in all patients after 4-6 weeks of medical treatment trial and avoidance of imaging during an acute upper respiratory tract infection is also advisable [4].
Since its development in the 1980s in Germany image guidance immerged a new platform for otolaryngologist. Contiguous 1mm thickness CT scan slices with 3-D reconstruction aids in diagnosing and providing accurate localization of bony partitions and boundaries [5,6]. In a study conducted by Loehrl et al. 31 patients underwent revision endoscopic frontal sinus surgery with image guidance with an average 11.9 months follow up. The authors commended that integrated instruments make revision frontal sinus surgeries efficient by utilizing the instrument after immediate localization has been accomplished [7].
Such accuracy is demonstrated in the illustrated cases in Figure 1 & 2 where in Figure 1 the navigation aided in surgeon’s advancement in the narrow areas between the orbit and skull base easing the technical challenge. And Figure 2 shows precise localization of the integrated probe touching the frontal sinus posterior wall. The benefit of image guidance can help in the diagnosis of the causes of frontal outflow blockage. In literature recurrent frontal disease can have variable causes highest being incomplete removal of uncinate process or air cells, iatrogenic injury or recurrent mucosal edema or frontal ostium stenosis [8].
Where is Valdes et al reported the findings in recurrent frontal disease in 66 patients to be hypertrophic mucosa (92.7%); retained agger nasi cell (73.4%); neo-osteogenesis within the frontal recess (45.9%); lateral scarring of the middle turbinate (47.7%); residual anterior ethmoid air cell (32.1.%); and residual frontal cells (24.8%) [9].
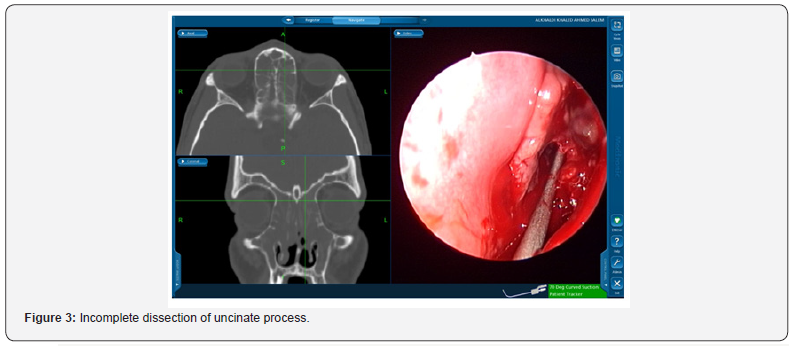
In the 60 patients reviewed the most common cases of frontal sinostomy failure were due to retained uncinate process, residual agger nasi with/without ethmoid disease (33%). Figure 3 & 4 display cases of retained uncinate process and undissected frontal air cell, respectively. Bradely et al. [10] recognized agger nassi cell in 93% of CT scan slides of their revision functional endoscopic sinus patients [10]. Whereas Nakayama et al also concluded through CT scan slide revision that residual frontal recess cells were independent risk factors for postoperative frontal sinusitis [11].
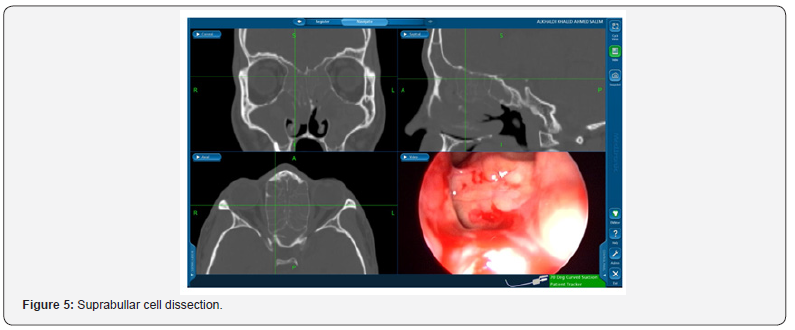
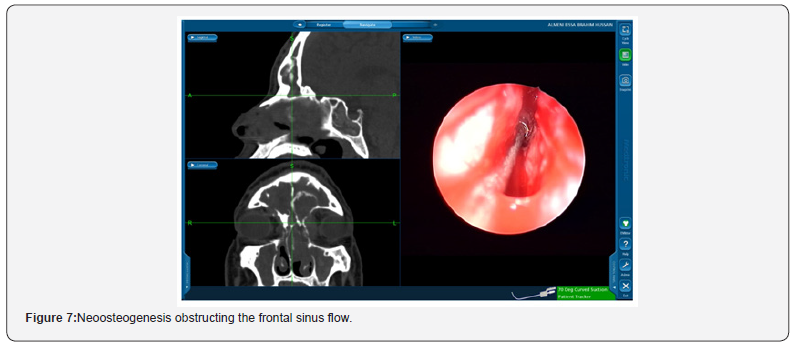
Suprabullar cells are also a recognizable cause of recurrence where is can be wronged for base of skull during primary surgery and left untouched [12]. That represents 11% of the findings in the revision cases in this study such as the illustrated case in Figure 5. In another recognizable cause 24% of the revised patients had lateralization of the middle turbinate Figure 6. This lateralization was found to be due to scarring between the turbinate and retained air cells or uncinate process 13%, 11% respectively. Other causes include neo-osteogenesis, which believed to be caused by failure to preserve normal mucosa [13]. Neo-osteogenesis as seen in Figure 7 presented 5% of our findings.
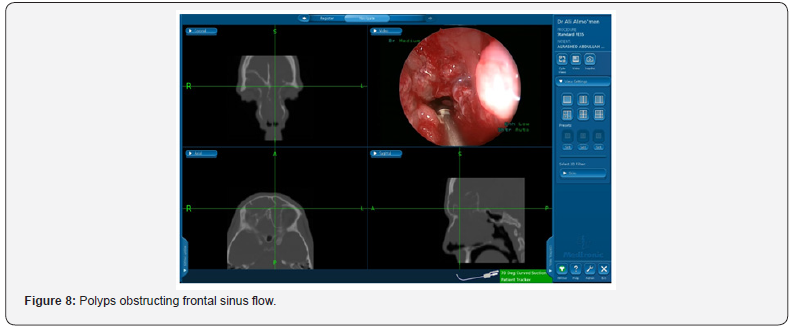
Even recurrence of the mucosal disease itself that is seen in Figure 8 presented as a cause of recurrence in 25% of our revised cases. Although higher rates have been reported in literature where Valdes et al. [9] reported a rate as high as 92.7% of edematous or hypertrophic mucosa found in CT scan of revised cases.
Conclusion
In conclusion, frontal sinus outflow presents a challenging technical surgery for all otolaryngologists. That difficulty is magnified in revision cases where anatomy is distorted and recognition of causes of failure is demanding. Image guidance is prized instrument in preforming such cases. Thorough dissection of all remnant bony partitions in the frontal recess is achievable with the assistance of image guidance. Achieving maximum drainage with preservation of vital structures is easily attained with accurate localization provided with image guidance.
References
- Musy PY, Kountakis SE (2004) Anatomic findings in patients undergoing revision endoscopic sinus surgery. Am J Otolaryngol 25(6): 418‐422.
- Schaitkin B, May M, Shapiro A, Fucci M, Mester SJ (1993) Endoscopic sinus surgery: 4-year follow-up on the first 100 patients. Laryngoscope 103(10): 1117‐1120.
- Khalil HS, Eweiss AZ, Clifton N (2011) Radiological findings in patients undergoing revision endoscopic sinus surgery: a retrospective case series study. BMC Ear Nose Throat Disord 11: 4.
- Kennedy DW, Senior BA (1997) Endoscopic sinus surgery. A review. Otolaryngol Clin North Am 30(3): 313-330.
- L Klimek, R Mösges, G Schlöndorff, W Mann (1998) Development of Computer-Aided Surgery for Otorhinolaryngology. Comput Aided Surg 3(4): 194-201.
- Bansal M (2000) Computer-assisted functional endoscopic sinus surgery (c-a fess) - a review. Indian J Otolaryngol Head Neck Surg 52(3): 311-314.
- Loehrl A, Toohill RJ, Smith TL (2000) Use of Computer‐Aided Surgery for Frontal Sinus Ventilation. Laryngoscope 110: 1962-1967.
- Orlandi RR, Kennedy DW (2001) Revision endoscopic frontal sinus surgery. Otolaryngol Clin North Am 34(1): 77‐90.
- Valdes CJ, Bogado M, Samaha M (2014) Causes of failure in endoscopic frontal sinus surgery in chronic rhinosinusitis patients. Int Forum Allergy Rhinol 4: 502-506.
- Bradley DT, Kountakis SE (2004) The Role of Agger Nasi Air Cells in Patients Requiring Revision Endoscopic Frontal Sinus Surgery. Otolaryngol Head Neck Surg 131(4): 525-527.
- Nakayama T, Asaka D, Kuboki A, Okushi T, Kojima H (2018) Impact of residual frontal recess cells on frontal sinusitis after endoscopic sinus surgery. Eur Arch Otorhinolaryngol 275(7): 1795‐1801.
- Kew J, Rees GL, Close D, Sdralis T, Sebben RA, et al. (2002) Multiplanar reconstructed computed tomography images improves depiction and understanding of the anatomy of the frontal sinus and recess. Am J Rhinol 16(2): 119‐123.
- McLaughlin RB Jr, Rehl RM, Lanza DC (2001) Clinically relevant frontal sinus anatomy and physiology. Otolaryngol Clin North Am 34(1): 1‐22.





























