Temporary Intubation to Manage Tracheomalacia Persisting After Teratoma Resection: A Case Report
Christopher Ian Newberry1*, Jonathan Skirko2 and J Fredrik Grimmer2
1The University of Utah, School of Medicine, Otolaryngology Head and Neck Surgery, Utah, US
2Primary Children’s Hospital, Pediatric Otolaryngology Head and Neck Surgery, Utah, US
Submission:December 12, 2019;Published:December 18, 2019
*Corresponding author:Christopher Ian Newberry, The University of Utah, Otolaryngology Head and Neck Surgery, Salt Lake City, Utah, US
How to cite this article:Christopher Ian Newberry, Jonathan Skirko, J Fredrik Grimmer. Temporary Intubation to Manage Tracheomalacia Persisting After Teratoma Resection: A Case Report. Glob J Oto, 2019; 21(3): 556064.DOI: 10.19080/GJO.2019.21.556064
Abstract
Tracheomalacia (TM) indicates a tracheal weakness with an increased collapsibility due to structural anomalies of the tracheal cartilage or posterior membrane. TM may be classified as primary-congenital or secondary-acquired disease. A rare cause of acquired TM is external tracheal compression. Pediatric TM can be challenging to treat, and numerous techniques have been described to manage the airway. This paper reports a severe case of acquired TM caused by a large cervicofacial teratoma managed with temporary intubation after tumor resection.
Keywords:Tracheomalacia; Tracheobronchomalacia; Airway collapse; Airway intervention; Teratoma
Introduction
Tracheomalacia (TM) is the most common congenital anomaly of the trachea.1 TM indicates tracheal weakness with an increased collapsibility due to structural anomalies of the tracheal cartilage or posterior membrane. This lack of rigidity results in an abnormal occlusion of the tracheal lumen and is classically defined as a reduction in cross-sectional area of at least 50% on expiration [1-3].
Symptoms typically arise acutely at birth or insidiously over the initial weeks to months [4]. Expiratory stridor and a barky/brassy cough are most common, but dysphagia, spontaneous neck hyperextension, recurrent respiratory tract infections, “death spells,” and respiratory distress with cyanosis and retractions are frequent presenting manifestations [1,5]. Symptoms are typically exacerbated by activities that increase respiratory effort, including coughing, feeding, and crying [2].
TM may be classified as primary (congenital) or secondary (acquired) disease [6]. Congenital TM is due to inadequate cartilage maturation, abnormal development of the cartilaginous matrix, or inherent tracheal weakness as in tracheoesophageal fistula (TEF) or esophageal atresia (EA). It can also be a feature of numerous other syndromes including mucopolysaccharidosis and CHARGE [2,6,7]. Acquired TM is caused by the degeneration of normal cartilaginous support [1]. This form commonly results from prolonged intubation and its associated ventilator requirements, tracheostomy, or external tracheal compression from vascular/cardiac anomalies or lesions such as tumors, cysts, abscesses, vascular malformations, and goiters [2].
Large cervical masses can distort the anatomy of the airway and compression may leave enduring effects, including TM. Even if the lesion does not cause persistent airway compression, it can collapse the airway during expiration, when there is increased intrathoracic pressure. Tracheal compression and recurrent collapse affect the integrity of the tracheal wall and increase the compliance of the disturbed portion [2]. Even after surgical correction of the compressive structure, focal tracheal weakness and collapse may persist. Pediatric TM can be challenging to treat, and numerous techniques have been described to manage the airway; however, there is no gold standard therapy. This paper reports a severe case of acquired TM caused by a large cervicofacial teratoma managed with temporary intubation after tumor resection.
Case Report
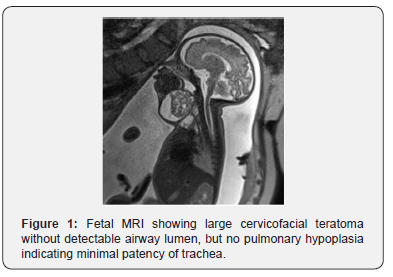
A female infant was prenatally diagnosed with a large cervicofacial teratoma at 32 weeks gestational age (GA) after her mother was found to be measuring large for dates, leading to an ultrasound followed by a fetal MRI (Figure 1). An ex-utero intra-partum therapy (EXIT) delivery was planned and executed at 35 weeks GA after spontaneous rupture of membranes with progression of labor. Intubation on placental circulation was attempted unsuccessfully. An urgent low tracheostomy was performed due to the mass inhibiting access to the proximal trachea.
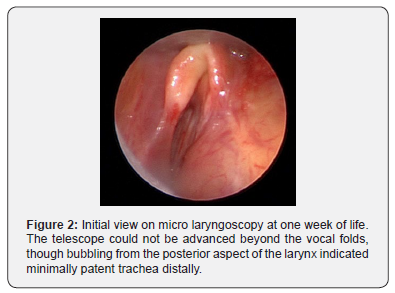
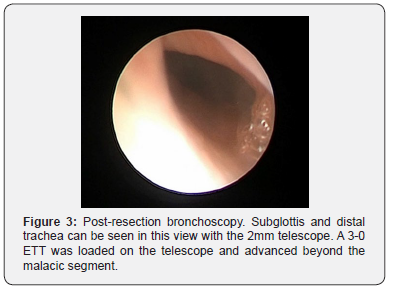
The infant was stabilized; however, over the next week, there was difficulty maintaining the tracheostomy tube due to distal granulation and frequent accidental decannulations. To evaluate the airway, micro laryngoscopy was performed at one week and showed severe external tracheal compression with inability to advance beyond the vocal folds (Figure 2). Teratoma resection was performed and repeat micro laryngoscopy and bronchoscopy (MLB) showed persistent severe tracheomalacia after resection (Figure 3). The tracheostomy tube was removed, and the patient was subsequently orotracheally intubated with a 3-0 endotracheal tube (ETT).
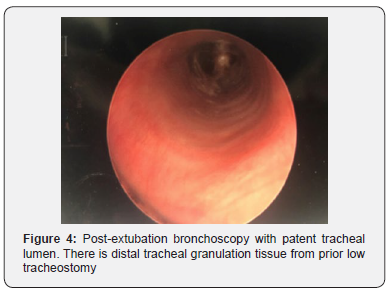
After one week of intubation, a repeat MLB was performed in the operating room. When the ETT was removed, there was no evidence of further collapse, with resolution of tracheomalacia (Figure 4). She was awoken from general anesthesia and returned to the neonatal intensive care unit extubated. Initially, she required supplemental oxygen delivered via high flow, which was weaned over two weeks. She was discharged from the hospital at five weeks of age without oxygen. Since discharge, she has had no episodes of respiratory distress, apnea, or cyanosis. At four months of age she is growing well, and trialing thickened oral feeds.
Discussion
Tracheomalacia presents a diagnostic and therapeutic challenge. Once diagnosed, treatment in the pediatric patient depends on the severity of airway collapse. In many children, intervention is avoidable. As the child grows, the tracheal cartilage strengthens making many cases of TM self-limited with spontaneous resolution by the second year of life [2,8]. However, although conservative treatment is often used in mild-to-moderate TM, severe or recalcitrant TM typically requires intervention.
For patients with symptomatic TM, available treatments include pharmacotherapy, positive pressure ventilation (CPAP), and various surgical interventions. Pharmacotherapy is indicated only for mild-to-moderate TM. These agents, such as bethanechol and ipratropium, can increase the tone of the trachealis muscle to decrease tracheal compliance [8]. CPAP may be trialed as a non-invasive measure; however, CPAP weakly stents the airway, delays oral feeding and speech, and is typically insufficient for severe cases [2]. When these non-invasive measures fail or are impractical, surgical measures are often required.
The current mainstays of surgical intervention include aortopexy, tracheostomy, resection, tracheoplasty, external splinting, and internal stents. Specific surgical treatment depends on the type, location, and severity of TM. Although short malacic segments are uncommon, resection with either end-to-end anastomosis or a slide tracheoplasty may be reasonable in these cases, especially if there is adjacent stenosis or other options are unfeasible. Unfortunately, these approaches are extremely invasive and no more than 30% of the pediatric trachea can undergo resection [1].
Traditional internal stents are frequently too large for use in neonates and are associated with numerous complications such as migration, granulation, infection, erosion, and mortality; therefore, they are typically recommended only in severe, refractory pediatric cases [9]. External splinting with autologous rib or prosthetic materials requires a thoracotomy or sternotomy and is best reserved for diffuse TM or localized TM (intra or extrathoracic) when other interventions are impractical [1,2,10,11]. Aortopexy has long been first-line surgical therapy for pediatric distal, intrathoracic TM. Aortopexy juxtaposes the aorta to the undersurface of the sternum, pulling the anterior tracheal wall forward and limiting intrathoracic tracheal collapse during exhalation. Again, this is an invasive procedure with significant mortality (6%) and complications (16.6%) and is not indicated in all-comers as severe focal collapse, extrathoracic TM, and diffuse weakness may persist [1,5,8].
Tracheostomy may be used to bypass severe disease restricted to the proximal trachea or to stent the middle/distal trachea. However, it is no longer routinely utilized as first-line treatment due its related complications and limitations. For example, tracheostomy is associated with predisposition for secondary TM, tracheal stenosis, granulation, recurrent infections, developmental delay, and respiratory arrest due to tube occlusion or decannulation [2,11]. Even more, children with large cervicofacial lesions have increased trachea to skin distance, difficult assess to the proximal trachea, displacement of the airway, and destabilized skin folds after resection. Consequently, orotracheal intubation or tracheotomy with retrograde intubation is typically preferred over tracheostomy [12]. Still, securing the airway at the time of delivery is of the utmost importance and low tracheostomy in these cases may be the only option until resection, as compression may limit the ability to pass an ETT. Thus, its primary utility in the management of TM is as a life-saving intervention in preparation for other management techniques when intubation is impossible, or if other interventions are impractical, tracheostomy may be used to bypass proximal disease until resolution of TM, knowing that this may take months to years [1,2].
Since each of these interventions has its disadvantages and limitations, they must be implemented only if absolutely indicated. For our case of extrathoracic, localized neonatal TM, reasonable surgical options included continued tracheostomy, external splinting, and perhaps resection. However, with large airway distorting lesions and subsequent severe TM, temporary intubation may be the first and only intervention required. Intubation is favored for securing the airway in the setting of large cervicofacial masses, is less invasive, and is now shown to be a possible intervention for TM following resection of an obstructing mass. Therefore, we recommend that temporary intubation after resection could be considered as the possible first step in management of TM in these patients (Figure 5).

The molding of the tracheal cartilage via intubation may take advantage of the cartilage’s plasticity also seen in neonatal auricular and nasal cartilage [13]. We suggest temporary intubation should be continued for at least 1-2 weeks in neonates and possibly most pediatric patients. In addition to avoid oxygen toxicity and high-pressure ventilation, the smallest ETT that adequately ventilates and gently stents should be used as this circumvents situations associated with predisposition to secondary TM [2]. After 1-2 weeks, once the patient is stable on extubatable settings, extubation with subsequent bronchoscopy may be trialed. If severe, symptomatic TM persists despite trial of temporary intubation, this may serve as an indication for further surgical intervention.
Conclusion
Acquired TM secondary to obstructing head and neck lesions can complicate patient care. These patients require intervention of the mass, establishment of an airway, and possible treatment of acquired TM. To establish an airway after delivery in a patient with an obstructing head and neck mass, direct orotracheal intubation or tracheotomy with retrograde intubation should be performed, if possible. If acquired TM is encountered after resection of the mass, temporary intubation may serve as the only intervention required.
References
- Fraga JC, Jennings RW, Kim PC (2016) Pediatric tracheomalacia. Semin Pediatr Surg 25(3): 156-164.
- Carden KA, Boiselle PM, Waltz DA, Ernst A (2005) Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest 127(3): 984-1005.
- Tan JZ, Ditchfield M, Freezer N (2012) Tracheobronchomalacia in children: review of diagnosis and definition. Pediatr Radiol 42(8): 906-915; quiz 1027-1028.
- Wittenborg MH, Gyepes MT, Crocker D (1967) Tracheal dynamics in infants with respiratory distress, stridor, and collapsing trachea. Radiology 88(4): 653-662.
- Kugler C, Stanzel F (2014) Tracheomalacia. Thorac Surg Clin 24(1): 51-58.
- Boogaard R, Huijsmans SH, Pijnenburg MW, Tiddens HA, de Jongste JC, et al. (2005) Tracheomalacia and bronchomalacia in children: incidence and patient characteristics. Chest 128(5): 3391-3397.
- Deacon JWF, Widger J, Soma MA (2017) Paediatric tracheomalacia - A review of clinical features and comparison of diagnostic imaging techniques. Int J Pediatr Otorhinolaryngol 98: 75-81.
- Hysinger EB, Panitch HB (2016) Paediatric Tracheomalacia. Paediatr Respir Rev 17: 9-15.
- Nicolai T (2008) Airway stents in children. Pediatr Pulmonol 43(4): 330-344.
- Morrison RJ, Sengupta S, Flanangan CL, Ohye RG, Hollister SJ, et al. (2017) Treatment of Severe Acquired Tracheomalacia With a Patient-Specific, 3D-Printed, Permanent Tracheal Splint. JAMA Otolaryngol Head Neck Surg 143(5): 523-525.
- Morrison RJ, Hollister SJ, Niedner MF, et al. (2015) Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci Transl Med 7(285): 285ra264.
- Walz PC, Schroeder JW, Jr (2015) Prenatal diagnosis of obstructive head and neck masses and perinatal airway management: the ex utero intrapartum treatment procedure. Otolaryngol Clin North Am 48(1): 191-207.
- Cottler PS, McLeod MD, Payton JI, Pineros Fernandez A, Black JS (2017) Plasticity of Auricular Cartilage in Response to Hormone Therapy. Ann Plast Surg 78(6S Suppl 5): S311-S314.





























