Complex Auditory Brain Stem Response and Electroencephalography Findings in Stuttering Children
Amr Ahmed Othman1, Ahmed M Imamm2, Salwa Mourad3*, Hassan M Elnady4, Ahmed Borai5 and Abdelrahim Abdrabou Sadek6
11Lecturer of Pediatric Neurology and Psychiatry, Department of Pediatrics, Faculty of Medicine, Sohag University, Sohag, Egypt
22Professor of Phoniatric, Otolaryngology Department, Faculty of Medicine, Sohag University, Sohag, Egypt
33Assistant Professor of Audio-vestibular Medicine, Otolaryngology Department, Faculty of Medicine, Sohag University, Sohag, Egypt
44Assistant Professor of Neurology, Neurology Department, Faculty of Medicine, Sohag University, Sohag, Egypt
5Lecturer of Neurology, Neurology Department, Faculty of Medicine, Sohag University, Sohag, Egypt
6Professor of Pediatric Neurology and Psychiatry, Department of Pediatrics, Faculty of Medicine, Sohag University, Sohag, Egypt
Submission: October 10, 2019; Published: October 28, 2019
*Corresponding author: Mourad S, Audio-vestibular Unit, Department of E.N.T, Faculty of Medicine, Sohag University, Egypt
How to cite this article: Amr Ahmed Othman, Ahmed M Imamm, Salwa Mourad, Hassan M Elnady, Ahmed Borai, Abdelrahim Abdrabou Sadek. Complex Auditory Brain Stem Response and Electroencephalography Findings in Stuttering Children. Glob J Oto, 2019; 21(1): 556053. DOI: 10.19080/GJO.2019.20.556053
Abstract
Objective: To objectively evaluate the stuttering children via Complex-Auditory brain stem response (C-ABR) and Video electroencephalography (EEG).
Methods: Fifty-one children were included in this case control study and divided into two groups of 21 patients (case group) and 30 controls matched for age and sex. The age of patients ranges from 4 to 15 years and they were diagnosed as stuttering patients at Psychiatry Clinic, Pediatrics Department and Phoniatrics Unit, Sohag University Hospital, in the period from January 2018 to January 2019. All children were evaluated by auditory brain stem evoked potentials and electroencephalography.
Results: As regards the absolute latency of C-ABR, there was a significant prolongation of V, A and D waves latency values in stuttering children when compared to children with normal development(p<0.001), also E wave latency was prolonged(p =0.014).Theta and delta waves was the most dominant EEG rhythm among patient group with significant difference in comparison to control group in which fast wave activity was the dominant rhythm(p<0.001). Epileptiform activities were recorded in 40 % of stuttering subjects. Temporal cortical activities were the most common abnormal epileptic activities recorded in 20% of stuttering subjects with significant difference in comparison to control group p=0.003),these findings point to a possible role of an organic etiopathogenesis of stuttering.
Conclusion: C-ABR is very promising in evaluation of patients with stuttering, EEG findings point to a possible role of an organic etiopathogenesis of stuttering.
Keywords: Stuttering; CABR; electroencephalography
Introduction
Stuttering is a disorder of “selection, initiation, and execution of motor sequences necessary for fluent speech production (WHO, 2014). In the world, approximately four times as many men as women stutter, encompassing 70 million people worldwide or about 1% of the world’s population [1]. Stuttering is generally not a problem with the physical production of speech sounds or putting thoughts into words. Acute nervousness and stress do not cause stuttering, but they can trigger stuttering in people who have the speech disorder, and living with a stigmatized disability can result in anxiety and high allostatic stress load (chronic nervousness and stress) that reduce the amount of acute stress necessary to trigger stuttering in any given person who stutters, exacerbating the problem in the manner of a positive feedback system; the name ‘stuttered speech syndrome’ has been proposed for this condition [2,3]. Although the exact etiology, or cause, of stuttering is unknown, both genetics and neurophysiology are thought to contribute [4]. Stuttering seems to be genetically linked; pedigree studies and molecular-genetic analysis have yielded some very interesting results [5,6]. Recent studies have shown both structural and functional neurological differences in children who stutter [7].
Stuttering is a disorder of “selection, initiation, and execution of motor sequences necessary for fluent speech production (WHO, 2014). In the world, approximately four times as many men as women stutter, encompassing 70 million people worldwide or about 1% of the world’s population [1]. Stuttering is generally not a problem with the physical production of speech sounds or putting thoughts into words. Acute nervousness and stress do not cause stuttering, but they can trigger stuttering in people who have the speech disorder, and living with a stigmatized disability can result in anxiety and high allostatic stress load (chronic nervousness and stress) that reduce the amount of acute stress necessary to trigger stuttering in any given person who stutters, exacerbating the problem in the manner of a positive feedback system; the name ‘stuttered speech syndrome’ has been proposed for this condition [2,3]. Although the exact etiology, or cause, of stuttering is unknown, both genetics and neurophysiology are thought to contribute [4]. Stuttering seems to be genetically linked; pedigree studies and molecular-genetic analysis have yielded some very interesting results [5,6]. Recent studies have shown both structural and functional neurological differences in children who stutter [7].
Neurophysiological factors that are thought to contribute to stuttering include, gray and white matter differences [8], neural network connectivity differences [1,3], atypical lateralization of hemispheric functions [9], and white matter connections. Adolescents and young adults who stutter were found to have more white matter connections in the right hemisphere as compared with normally fluent controls [10]. There are some confirming reports about the role of EEG on the explanation of neurophysiology of stuttering. Various number of EEG studies suggested possible relationship between epileptic EEG activity and stuttering [11]. Most of these studies reported hemisphere asymmetry in stuttering patients; however, none of the EEG studies investigated regional differences, except neuroimaging and autopsy studies.
Complex auditory brain stem response (C-ABR) is a diagnostic tool that helps in explanation of neurophysiology of stuttering, it reflects speech encoding at the level of the brainstem by using consonant vowel stimuli to the ear.17 The response of the auditory brain stem to speech mimics the acoustic characteristics of the speech signal with apparent fidelity so it provides clinically applicable information about the auditory processing of complex stimuli [12]. The C-ABR provides representations of many aspects of the acoustic structure of speech, including separate neural representations of speech sound onset, phase-locking to the fundamental and formant frequencies of speech and speech sound offset [13]. Therefore, the rationale of our study is to use a combination of evoked potential, which is the C-ABR and the EEG to objectively evaluate the stuttering children and trying to know the pathogenesis of this disorder.
Patients and Methods
Participants and study design
Fifty-one children were included in this case control study and divided into two groups of 21 patients (case group) and 30 controls. The age of patients ranged from 4 to 15 years and they were diagnosed as stuttering patients at Psychiatry Clinic, Pediatric Department and Phoniatric Unit, in our University Hospital, in the period from January 2018 through January 2019. Exclusion criteria included patients with history of hearing loss, ear disease, trauma, and ototoxic drug intake or ear operations with normal middle ear functions as evidenced by otological examination, tympanometry and acoustic reflex thresholds which were done in Audiology Unit, in our University Hospital. Hearing threshold doesn’t exceed 20dBHL in the frequencies from 250Hz to 8000Hz. The patients did not receive language therapy.
The Control group included 30 children age and sex matched, without history of hearing loss or language or speech abnormalities. Normal middle ear functions as evidenced by otological examination, tympanometry and acoustic reflex thresholds. Threshold doesn’t exceed 20dBHL in the frequencies from 250Hz to 8000Hz. A written consent from all patients’ and controls’ parents/guardians was taken to approve sharing in the study after full description of the steps and aim of the study. The work has been carried out in accordance with the code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and was approved by the Ethical Committee of the Faculty of Medicine, Sohag University.
Methods
All patients were subjected to stuttering severity assessment at Phoniatrics Unit by use of stuttering severity index third edition (SSI-3) [14]. Stuttering Severity Instrument includes three parameters:
a. Frequency of repetitions and prolongation of sounds and syllables.
b. Estimated duration of the longest stuttering events.
c. Observable physical concomitants.
The SSI-3 test record and frequency computation form are divided into 4 major areas:
i. Frequency (converted to scale score from 0 through 18).
ii. Duration (converted to scale score from 0 through 18).
iii. Physical concomitants (rated by degree of distractibility 0 through 20).
iv. Severity conversion tables for preschool children, school-age children, and adults.
By adding the score of the three parameters (frequency, duration and physical concomitants), a total overall score is obtained. The severity of stuttering, as measured by these parameters, can be ascertained by comparing this score to the age appropriate normative data for preschool children, school age children and adults. The score can be described as a percentile, or by an adjective that describes the level of stuttering severity as very mild, moderate, severe or very severe.
All children were assessed through the audiology department as follow,
a) Equipment’s: Sound treated room IAC model 1602, Pure tone audiometry: Madsen Orbiter 922, Immittancemetry: Maico MI44, Evoked potentials system SMART intelligent hearing system.
b) Procedure: All subjects were subjected to 1-Informed written consent from parents. 2-Full history taking. 3-Otological examination. 4-Basic audiological evaluation: Pure tone audiometry including air and bone conduction, speech audiometry including: -Speech Reception Threshold (S.R.T): using Bisyllabic words for children [15].
c) Speech Discrimination (S.D): using Arabic Phonetically balanced Kindergarten (PBKG) words [15]. 5- Immittancemetry including tympanometry and acoustic reflex threshold. 6-Click evoked Auditory Brainstem Response: to confirm presence of wave V.
d) Stimulus Parameters: type: click stimulus, intensity: 90dBnHL, polarity: alternating, Presentation rate: of 13.1 p/sec, mode of delivery: stimuli were presented monaurally to the right ear via an ER3A- insert phone. Recording parameters: electrode montage: The active electrode was placed on the high frontal (Fz), the ground electrode on the low frontal (FPz), the negative electrode on the right side and the reference electrode on the left side. Number of sweeps: 1024, filter: band passes of 100 to 1500 Hz, analysis period: 0 to 12 msec.
Complex Auditory Brainstem Response (C-ABR)
a) Stimulus Parameters: Type: 40-ms /da/ syllable it consists of onset noise burst during the first 10 ms and formant transition between the consonant and a steady-state vowel. The stimulus was generated by Intelligent Hearing System Company and included in speech auditory brain response software. Intensity: 80 dBSPL, polarity: alternating, presentation rate: of 11p/sec, mode of delivery: stimuli were presented monaurally to the right ear via an ER3A- insert phone. Recording parameters: Electrode montage: The active electrode was placed on the high frontal (Fz), the ground electrode on the low frontal (FPz), the negative electrode on the right side and the reference electrode on the left side.
There are no ear differences in complex ABR, so the recordings were obtained from the right ear only [16]. All electrodes were connected to the pre-amplifier of the Smart EP equipment. Number of sweeps: 4000, filter: band passes of 100 to 1500Hz, analysis period: 75msec including 15msec pre-stimulus recording. Response analysis: The response was identified by the presence of seven waves (V, A, C, D, E, F, O), wave V analogous to the wave V elicited by click stimuli, followed immediately by a negative trough (wave A). Following the onset response, a series of peaks (C to F) represent FFR. Offset response is represented by wave O. The wave’s absolute latency, VA amplitude were measured.
All children were subject to electroencephalography (EEG) as per American Clinical Neurophysiology Society guidelines using 16 channel RMS computerized EEG machine for a minimum of 40 minutes to capture both wakefulness and sleep along with activation procedures like hyperventilation (if feasible) and photic stimulation. EEG was reviewed for the dominant EEG rhythm and EEG epileptiform activities.
Statistical Analysis
Data was analyzed using IBM SPSS Statistics for Windows version 23.0. Quantitative data was expressed as means ± standard deviation, median and range. Qualitative data was expressed as number and percentage. The data were tested for normality using Shapiro-Wilk test. The nonparametric Mann–Whitney test, Kruskal–Wallis test were used for data which wasn’t normally distributed. One-Way ANOVA test were used for normally distributed data. Chi-Square test was used for comparison between qualitative variables. A 5% level was chosen as a level of significance in all statistical tests used in the study.
Results
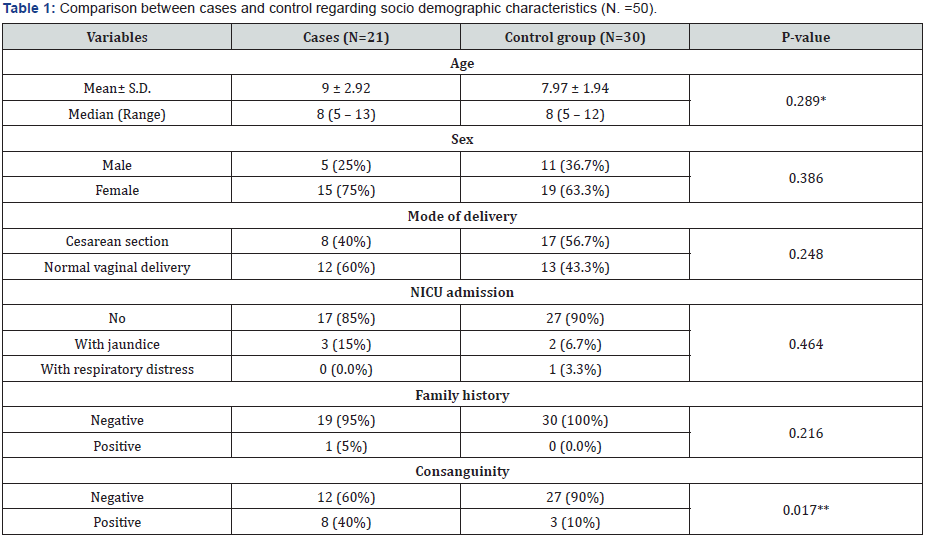
P- value was calculated by Chi square test
*P- value was calculated by Mann-Whitney U test
**P- value was calculated by Fisher’s Exact Test
P-value < 0.05 is statistically significant
Fifty one children were included in this case control study and divided into two groups of 21 patients (case group) and 30 controls with comparable age and sex with no significant difference, respectively (p= 0.289, p= 0.386).The most common mode of delivery among cases was normal vaginal delivery (60%) and the most common mode of delivery among controls was cesarean section (56.7%) with no significant difference (p=0.248). Most of cases and controls had no history of NICU admission, respectively (85%, 90%) with no significant difference (p= 0.464). As regard family history of stuttering among cases and controls, respectively (5%, 0.0%) with no significant difference (p= 0.216). Incidence of consanguinity among cases and controls was respectively (40%, 10%) with significant difference (p=0.017) (Table 1).
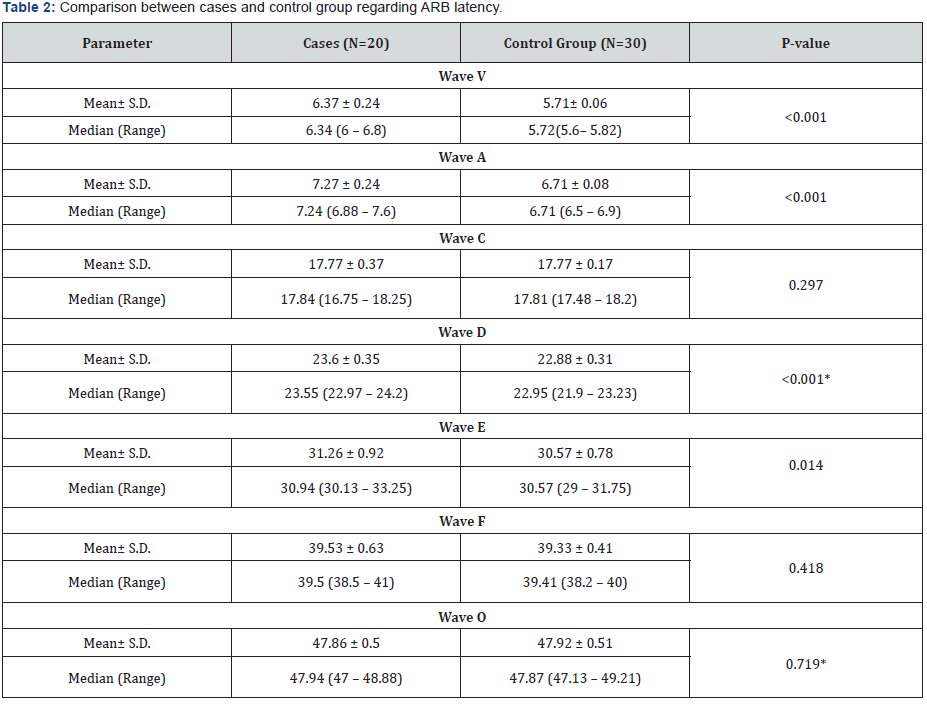
P- value was calculated by Mann-Whitney U -test.
*P- value was calculated by Independent Samples test.
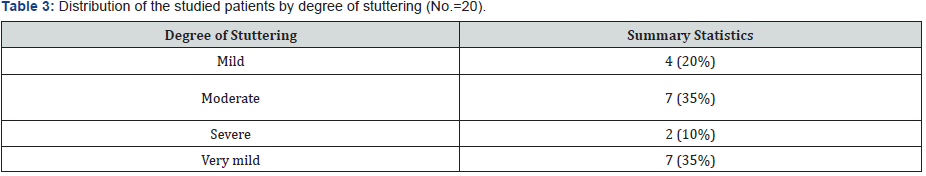
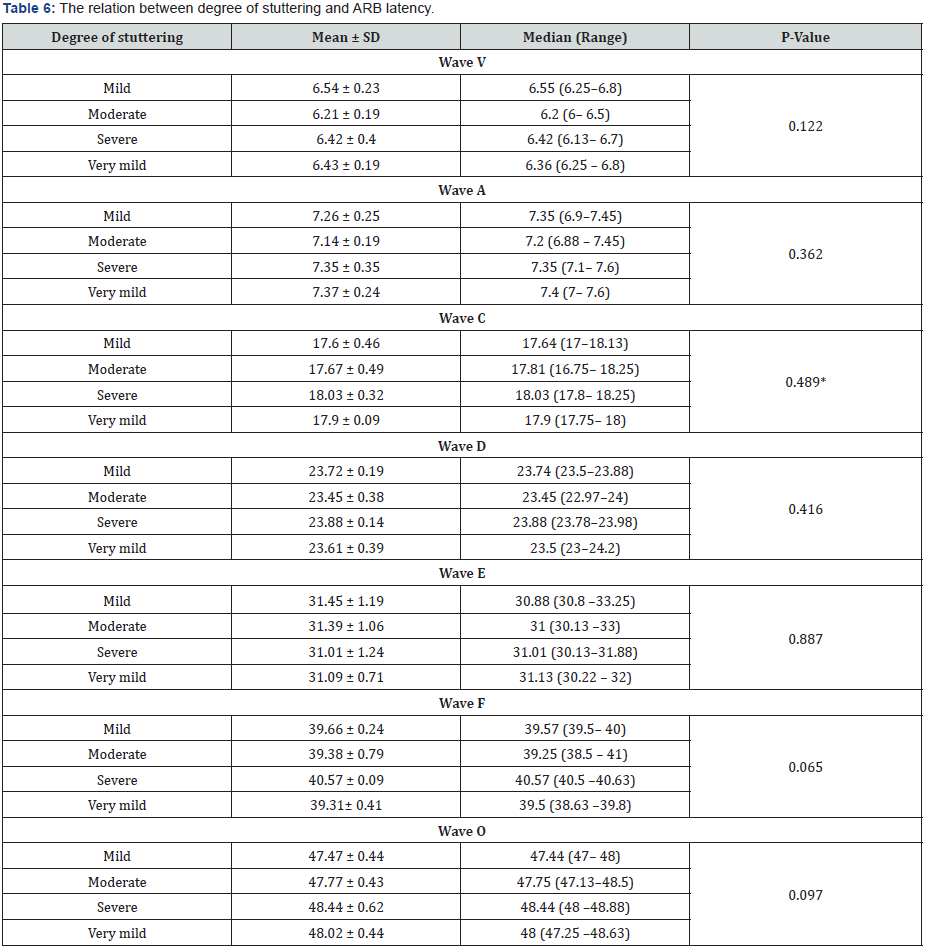
Audiological evaluation showed that in comparison to control group, ABR latency of wave V (6.37 ± 0.24), wave A (7.27 ± 0.24) and wave D (23.6 ± 0.35) were detected in patients’ group with significant delay (p<0.001). ABR latency of wave E in patients’ group was also delayed in comparison to control group (31.26 ± 0.92 vs. 30.57 ± 0.78) (p= 0.014). However, ABR latency of wave C, F and O in patients group show no significant difference in comparison to control group (p=0.297, p=0.418 and p= 0.719 respectively) (table 2). Assessment of stuttering degree in patients group revealed moderate degree of stuttering in seven cases (35%), also seven cases (35%) had very mild degree followed by mild degree in four cases (20%) and severe degree in two cases (10%) (Table 2 & 3)
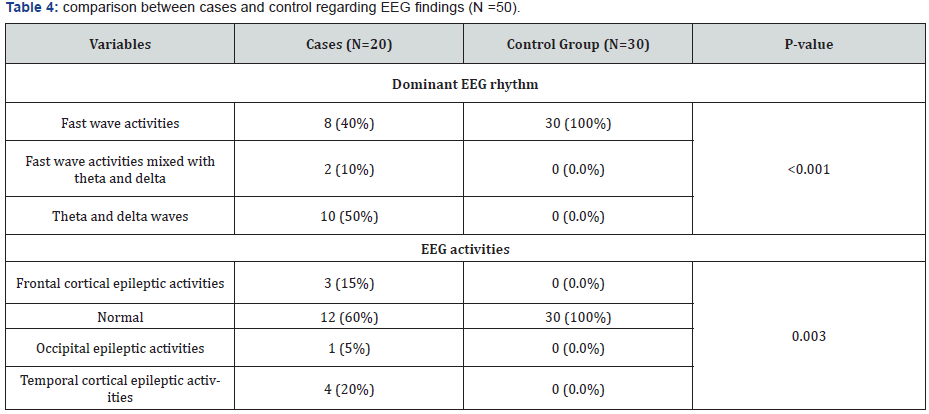
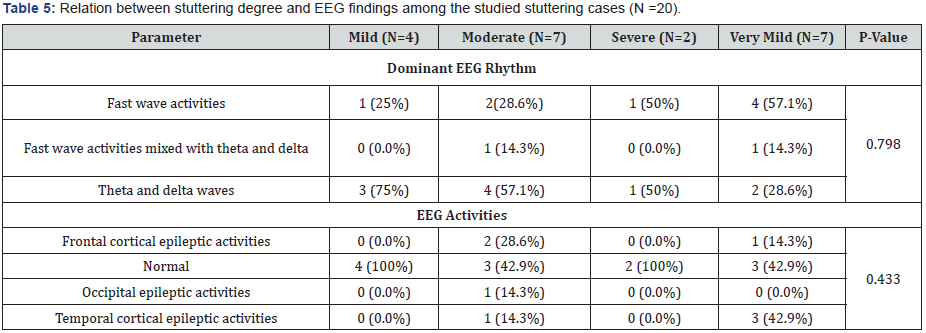
Theta and delta waves were the most dominant EEG rhythm (50%) among patients group followed by fast wave activities and fast wave activities mixed with theta and delta waves (40% and 10%) respectively with significant difference in comparison to control group in which fast wave activity was the most dominant rhythm (100%) (p<0.001). As regard epileptic EEG activities; normal EEG record was the most common EEG finding in 12 cases (60%) followed by Temporal , Frontal and Occipital epileptic activities (20%, 15% and 5%) respectively with significant difference in comparison to control group in which normal EEG record was the most common EEG finding in all cases (100%) (p=0.003) (Table 4).
P- value was calculated by Chi square test.
As regard EEG rhythm in patients’ group, theta and delta waves was the most dominant rhythm in patients with mild, moderate, severe and very mild degree of stuttering (75%, 57.1%, 50% and 28.6%) respectively with no significant difference in comparison to other EEG rhythms(p=0.798). Normal EEG record was the most common EEG activity in patients with mild, moderate and severe degree of stuttering( 100%, 42.9% and 100%) respectively, while normal EEG record was equal to Temporal cortical activities (42.9%) in patients with very mild degree of stuttering with no significant difference in comparison to other EEG activities(p=0.433) (Table 5 & 6).
P- value was calculated by One Way ANOVA test.
*P- value was calculated by Kruskal Wallis Test.
P- value was calculated by One Way ANOVA test.
*P- value was calculated by Kruskal Wallis Test.
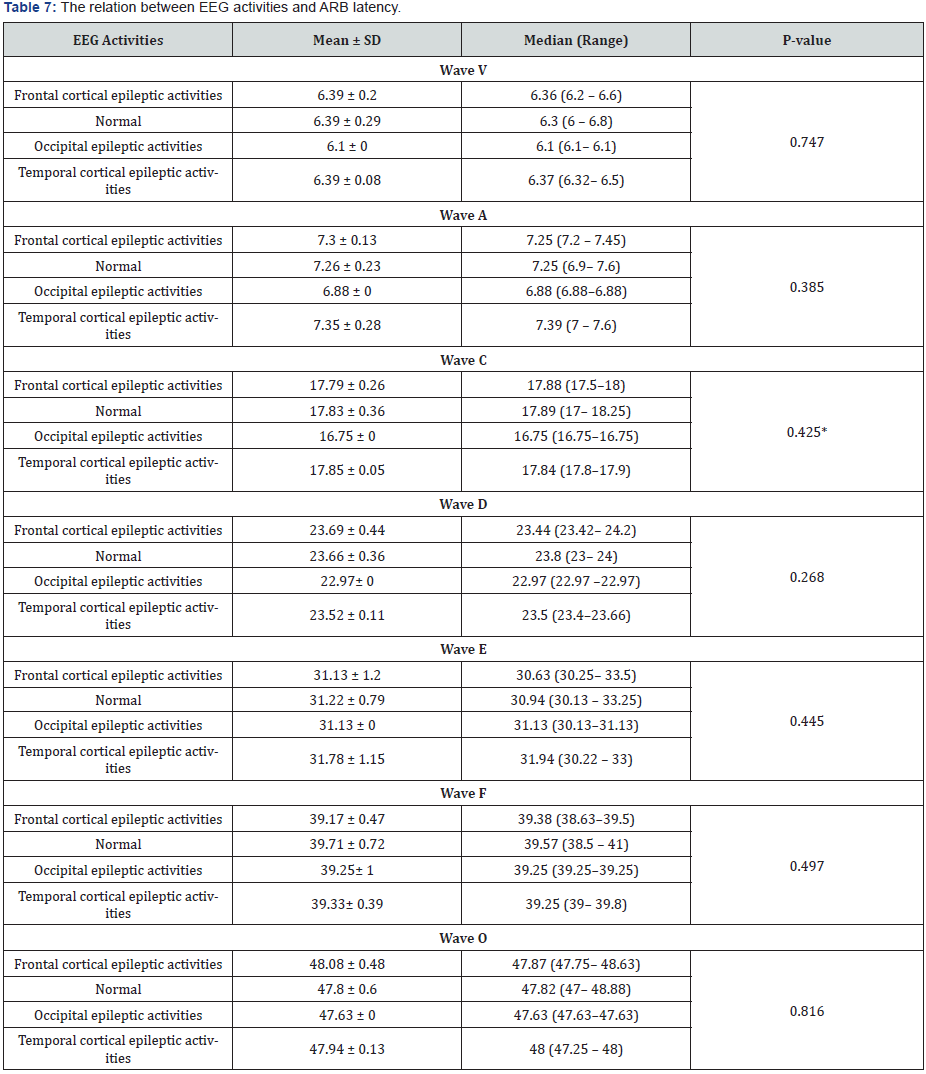
Also, in comparison between ABR latency of different waves (V, A, C, D, E, F, O) with different EEG activities, no significant difference was noticed (Table 7).
Discussion
Although the exact pathogenesis of stuttering is unknown, both genetics and neurophysiological factors are contributing. Various number of EEG study suggested possible relationship between epileptic EEG activity and stuttering [11]. In addition, the neurophysiology of stuttering can be explained by complex auditory brain stem response (C-ABR) [13].
In our study, the whole seven waves of C-ABR were identified in all participants (Table 1). This agrees with a study done on 88 normal adults by Johnson et al and they reported that the waves were present in 100% of the individuals [17]. While our results disagree with [18], who studied C-ABR in normal individuals and they concluded that the identification of the onset peaks (V and A) were 100%,while it was 87 % for wave D, 91 % for wave E, 91.6 for wave F, 83.3 % for wave O (83.3%) and only 66 for wave C [18]. The differences between the results of the two studies could be attributed to the smaller sample in the Hornickel [18], study, only twelve subjects.
As regards the absolute latency of C-ABR, there was a statistically significant difference in C-ABR latencies between control and patients’ group in V, A and D waves (Table 1). This agrees with findings observed by [19,20], who described a difference between the V and A wave latency values in stuttering children when compared to children with normal development. Prolonged absolute latencies of waves V and A suggest the existence of functional impairment in speech processing in the brainstem region. It suggests also, alteration of the physiological mechanisms, even without a proven neurobiological abnormality in stuttering children.
This functional impairment in the speech perception is the leading cause of the impairment of the communication and language processes in stuttering children with consequent degradation of linguistic and paralinguistic information. Thus, there is a negative impact on the cortical processing of speech signals. These cortical areas do not respond to the speech stimulus in a synchronous and organized fashion and thus impaired the interpretation and understanding of speech in stuttering children. This can result in severe consequences in social interactions and the quality of life.
In the current study there were no statistically significant differences in the absolute latency of waves E, F & O (Table 1). This agrees with a study done by Milaine [21], this suggests the encoding of speech sounds in the subcortical areas i.e. lateral lemniscus and inferior colliculus.
In our study theta and delta waves was the most dominant EEG rhythm (50%) among patients’ group with significant difference in comparison to control group in which fast wave activity was the dominant rhythm (100%) (Table 3). This agrees with finding observed by [22,23] who described that the dominant EEG rhythm was slower in stutterers compared with the control group. In our study epileptiform activities were recorded in 40 % of stuttering subjects. Temporal cortical activities were the most common abnormal epileptic activities recorded in 20% of stuttering subjects with significant difference in comparison to control group (Table 3). These findings point to a possible role of an organic etiopathogenesis of stuttering, that agree with findings recorded by [22,23].
There was no statistically significant difference in EEG findings in different degrees of stuttering. This means that different EEG rhythms and activities can be observed in patients with stuttering whatever its degree. Also, there were no statistically significant differences in C-ABR in different degrees of stuttering which means that stuttering can affect the C-ABR response whatever its degree.
Finally, in our study there were no statistically significant differences between C-ABR latency and EEG activities. This is true as the auditory evoked potentials are like a brain wave test (EEG) except that the measured EEG response was done to a special auditory stimulus during the examination [24].
C-ABR is very promising in evaluation of patients with stuttering as it is practical, objective and not need the attention of the patient. However further studies with follow-up of these cases would be interesting to assess test accuracy and treatment effectiveness and to give a numerical analysis for monitoring the consequences of impaired speech perception in stuttering children. In the current study, we found a spectrum of abnormal EEG findings like abnormal EEG rhythm and different interictal epileptic EEG activities that point to the possible role of an organic etiopathogenesis of stuttering. Although, there is no specific pattern of EEG abnormalities associated with stuttering in children, we recommend a routine EEG in all these children. Further, researches are needed to unravel the electroencephalographic basis of stuttering and to provide insight into a specific pharmacologic treatment of stuttering [25].
Conclusion
Audiological evaluation has an important role in the evaluation of stuttering children. ABR latency of waves V, A, D, E was detected with significant delay in those patients. More than two thirds of our patients have very mild and moderate degree of stuttering. Theta and delta waves were the most dominant EEG rhythm in stuttering children in addition normal EEG findings was the most common in those patients.
References
- Carlson N (2013) Human Communication. In Physiology of behavior, (11th edn), Boston: Allyn and Bacon, pp. 497-500.
- Irwin M (2006) Terminology – How should stuttering be defined? And why? – Research, Treatment, and Self-Help in Fluency Disorders: New Horizons. In: Au Yeung J, Leahy MM (Eds.), The International Fluency Association. pp. 41-45.
- Bowen, Caroline (2015) Information for Families: Stuttering- What can be done about it? Speech-language-therapy dot com. Archived from the original on April 2, Retrieved June 19, 2013.World Health Organization ICD-10 F95.8 – Stuttering Archived at the Wayback Machine.
- Ashurst JV, Wasson MN (2011) Developmental and persistent developmental stuttering: an overview for primary care physicians. The Journal of the American Osteopathic Association 111 (10): 576-580.
- Ghiselli N, Davis G (2011) Stuttering: Fact or fiction? PsycCRITIQUES 56(13).
- Fedyna A, Drayna D, Kang C (2011) Characterization of a mutation commonly associated with persistent stuttering: evidence for a founder mutation. J Hum Genet 56(1): 80-82.
- Chang SE (2014) Research updates in neuroimaging studies of children who stutter. Seminars in Speech and Language 35: 67-79.
- Chang SE, Erickson KI, Ambrose NG, Hasegawa Johnson MA, Ludlow CL (2008) Brain anatomy differences in childhood stuttering. Neuroimage 39(3): 1333-1344.
- Weber Fox C, Wray AH, Arnold H (2013) Early childhood stuttering and electrophysiological indices of language processing. Journal of Fluency Disorders 38(2): 206-221.
- Watkins KE, Smith SM, Davis S, Howell P (2008) Structural and functional abnormalities of the motor system in developmental stuttering. Brain 131(1): 50-59.
- Nuwer M (1997) Assessment of digital EEG, quantitative EEG, and EEG brain mapping: Report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology 49: 277-292.
- Prestes R, de Andrade AN, Santos RB, Marangoni AT, Schiefer AM, et al. (2017) Temporal processing and long latency auditory evoked potential in stutterers. Braz J Otorhinolaryngol 83(2): 142-146.
- Jansson Verkasalo E, Eggers K, Järvenpää A, Suominen K, Van den Bergh B, De Nil L, et al. (2014) Atypical central auditory speech-sound discrimination in children who stutter as indexed by the mismatch negativity. J Fluency Disord 41: 1-11.
- Bakhtiar M, Seifpanahi S, Ansari H, Ghanadzade M, PackmanA (2010) Investigation of the reliability of the SSI-3 for preschool Persian-speaking children who stutter. J Fluency Disord 35(2): 87-91.
- Soliman S, Shehata W, Fatthallah A (1985) Development of Arabic Staggered Spondee Words (SSW). Paper presented at the 8thAin Shams Annual Congress 2: 1220-1246.
- Vander W, Kathy R (2011) Brain Stem Responses to Speech in Younger and Older Adults. EAR &HEARING 32(2): 168-180.
- Johnson KL, Nicol TG, Kraus N (2005) Brain stem response to speech: A biological marker of auditory processing. Ear Hear 26: 424-434.
- Hornickel J, Skoe E, Kraus N (2009) Subcortical laterality of speech encoding. Audiol Neurootol 14: 198-207.
- Abrams DA, Nicol T, Zecker SG, Kraus N (2010) Rapid acoustics processing in the auditory brainstem is not related to cortical asymmetry for the syllable rate of speech. Clin Neurophysiol 121: 1343-1350.
- Skoe E, Kraus N (2010) Auditory brainstem response to complex sounds: a tutorial. Ear Hear 31(3): 302-324.
- Sanfins MD, Borgesa LR, Ubialia T, Santosb MFC (2017) Speech auditory brainstem response (speech ABR) in the differential diagnosis of scholastic difficulties Brazilian. Journal of Otorhinolaryngology 83(1): 111-116.
- Khedr E, El Nasser WA, Abdel Haleem EK, Bakr MS, Trakhan MN (2000) Evoked potentials and electroencephalography in stuttering. EEG Abnormalities in Children with Speech and Language Impairment. Folia Phoniatr Logop 52(4): 178-86.
- Bharati Mehta, VK Chawla, Manish Parakh, Poonam Parakh, Bharti Bhandari, et al. (2015) EEG Abnormalities in Children with Speech and Language Impairment. J Clin Diagn Res 9(7): CC04-CC07.
- Jewett DL, Williston JS (1971) Auditory evoked far fields averaged from the scalp of humans. Brain 94: 681-696.
- Chang SE, Zhu DC (2013) Neural network connectivity differences in children who stutter. Brain 136(12): 3709-3726.





























