Novel Spectrophotometric Methods for Simultaneous Determination of Cefixime trihydrate and Sodium benzoate in Powder for Oral Suspension Dosage form
Mohamed EM Hassouna1*, Maha M Abdelrahman2 and Mahmoud A Mohamed3
1Department of Chemistry, Beni-Suef University, Egypt
2Department of Pharmaceutical Analytical Chemistry, Beni-Suef University, Egypt
3Department of Quality Control, Hikma Pharmaceutical Company, Egypt
Submission: December 14, 2017; Published: December 22, 2017
*Corresponding author: Mohamed EM Hassouna, Department of Chemistry, Beni-Suef University, Egypt, Tel: +2 01223861504; Email: mhassouna47@hotmail.com; Mohamed.hassona@science.bsu.edu.eg
How to cite this article: Hassouna MEM, Abdelrahman MA, Mohamed MA. Novel Spectrophotometric Methods for Simultaneous Determination of Cefixime trihydrate and Sodium benzoate in Powder for Oral Suspension Dosage form. Glob J Oto 2017; 12(4): 555841. DOI: 10.19080/GJO.2017.12.555841
Abstract
Cefixime is generally classified as a third-generation cephalosporin antibacterial and is given orally to treat infection due to susceptible gram-positive and gram-negative bacteria. Up till now, no spectrophotometric methods have been published for the determination of cefixime trihydrate and sodium benzoate in their pharmaceutical formulation. Novel, specific, precise, simple, and accurate spectrophotometric methods are developed and validated for the simultaneous determination of cefixime trihydrate (CFX) and sodium benzoate (SDB) in powder for oral suspension (POS) dosage form. Firstly, CFX is directly determined using its extended spectra at 290 nm, where no interference from the coformulated SDB has been detected. In method (I), CFX and SDB are determined by first derivative ratio spectrophotometric method (1DD) at 284 and 318 nm for CFX, while SDB is determined at 216 and 234 nm.
In method (II), simultaneous ratio subtraction method (SRSM) is established for resolving the overlap between CFX and SDB by dividing the spectrum of the binary mixture by the standard spectrum of 20 μg/mL CFX as adivisor then subtract the constant value determined in the plateau region at 240-325 nm, then multiply by the divisor to obtain zero order (D0) original spectrum of SDB at 227 nm, further zero order of CFX is determined at λmax 290 nm after multiplication the divisor by the obtained constant. Finally, in the third one; ratio difference spectrophotometric method (RDSM), laboratory prepared mixtures are divided by the absorption spectra of standard 40 μg/mL CFX for the determination of SDB and standard 30 μg/mL SDB for the determination of CFX. The ratio spectra are recorded at 231 nm and 290 nm for CFX, while at 223 nm and 250 nm for SDB. The obtained results for the proposed methods are statistically compared with those obtained by the official method for CFX besides the recently published HPLC one for their simultaneous determination using one-way analysis of variance (ANOVA) where no significant difference is observed between the proposed methods and the well-established ones which prove their validity for the analysis of this binary mixture.
Keywords: Cefixime Trihydrate; Sodium Benzoate; Zero order; First derivative ratio spectrophotometric method; Simultaneous ratio subtraction method; Ratio difference Spectrophotometric method
Abbreviations: CFX: Cefixime Trihydrate; SDB: Sodium Benzoate; SRSM: Simultaneous Ratio Subtraction Method; RDSM: Ratio Difference Spectrophotometric Method; ANOVA: Analysis of Variance
Introduction
Cefiximetrihydrate (CFX); is chemically known as (6R,7R)- 7-[[(Z)-2-(2-Aminothiazol-4-yl)-2-[(carboxymethoxy) imino] acetyl] amino]-3- ethenyl-8-oxo-5-thia-1-azabicyclo [4.2.0] oct- 2-ene-2-carboxylic acid trihydrate (Figure 1a) [1,2]. Cefixime is a bactericidal drug and is stable to hydrolysis by many beta-an antibacterial. Like other cephalosporin, it possesses a mechanism of action like penicillins i.e. inhibition of transpeptidation process resulting in the formation of imperfect cell wall; osmotic drive from the outside isotonic environment of the host cell to the inside of the hypertonic bacterial cytoplasm and finally activation of the autolysin enzyme leading to the lysis of bacteria. Only 40 to 50% of an oral dose of cefixime is absorbed from the gastrointestinal tract, whether taken before or after meals, although the rate of absorption may be decreased in the presence of food [3]. Cefixime is better absorbed from oral suspension than from tablets. Cefixime is generally classified as a third-generation cephalosporin antibacterial and is given orally to treat infection due to susceptible gram-positive and gram-negative bacteria, including gonorrhea and infections of the respiratory and urinary tracts [3].
Sodium benzoate (SDB); is chemically known as sodium benzene carboxylate (Figure 1b) [1,2].Sodium benzoate is used primarily as antimicrobial preservative in cosmetics, foods, and pharmaceuticals. It is used in concentration of 0.02-0.5% in oral medicines, 0.5% in parenteral products, and 0.1-0.5% in cosmetics. The inhibitory concentration of sodium benzoate required in emulsion increases with oil content. It has also been used as a tablet lubricant at 2-5 %w/w concentrations, providing rapid disintegration times [4]. Cefixime is official in British Pharmacopeia (BP), European Pharmacopeia (EP) [1,2] and United States Pharmacopeia (USP) [5], both of them includes HPLC method for estimation of CFX. Literature review revealed that various analytical methods have been described for the determination of cefixime including colorimetric and spectrophotometric [6-19], thin layer chromatography [20-23], capillary electrophoresis [24-26], HPLC [27-36] and electrochemical methods [37-40] in pure or in dosage forms.

Sodium benzoate is official in BP, EP [1,2] and United States Pharmacopeia (USP) [5], which includes direct titration for the estimation of SDB. Thus, a variety of methods have been developed for its determination including spectrophotometric [41,42], thin layer chromatography [43] and HPLC [44-53]. Recently, an HPLC method [54] has been published for simultaneous determination of both CFX and SDB together in pure or dosage forms. The combination of these two drugs is not official in any pharmacopoeia. According to the best of our knowledge, no spectrophotometric method has been published for determination of both CFX and SDB together in pure or dosage forms. The present work aims to develop novel spectrophotometric methods, for the first time, for the determination of CFX and SDB in pure and in their dosage form with high sensitivity and low cost. The developed methods have advantages over the published ones regarding simplicity and no need for expensive or sophisticated apparatus. Besides, they are suitable as routine methods in quality control analysis laboratories. They are validated according to ICH guidelines.
Materials and Methods
Apparatus
UV- 1800 double beam UV-Visible spectrophotometer (Shimadzu-Japan) with highest resolution in which the spectral bandwidth is 1nm for the spectral range 190-1100 nm, is used for all absorbance measurements, matched with 1 cm quartz cells. Data analysis is performed by software (UV-Probe 2.5.2).
Pure samples
Pure samples of cefixime trihydrate and sodium benzoate are kindly supplied by EPCI Pharmaceutical Company part of HIKMA group, Beni-Suef, Egypt with claimed purity of 99.97% and 99.90%, respectively, according to quality control certificates of analysis.
Pharmaceutical dosage form
SUPRAX® 100 mg/5mL POS (60mL) (Batch No. 2000) and SUPRAX®100 mg/5mL POS (30 mL) (Batch No. 2002) are manufactured by EPCI Pharmaceutical Company part of HIKMA group, Beni-Suef, Egypt. Each 5mL is claimed to contain 100 mg of cefixime and 2.5 mg of sodium benzoate.
Chemicals
Methanol of HPLC-grade is used as a solvent. It is obtained from (Scharlau, Spain).
Preparation of standard and samples Solution
Stock Solutions of Cefixime and Sodium Benzoate (1000 μg /mL): Accurately weigh 100mg of each of CFX & SDB, transfer into 100 mL volumetric flask, add 70 mL of the solvent and sonicate to dissolve and complete to the mark with the same solvent and mix well.
Working Standard Solutions of Cefixime and Sodium Benzoate (100μg /mL): Accurately transfer 10 mL of CFX & SDB from their stock solutions in two 100 mL volumetric flasks, add 70 mL of the solvent and sonicate to dissolve and complete to the mark with the same solvent and mix well.
Laboratory Prepared Mixture: Prepare mixtures of CFX & SDB containing different ratios from their working standard solutions (100 μg /mL) into a series of 10 mL volumetric flasks. Make up to the mark with the same solvent. concentrations of CFX & SDB are calculated from the corresponding regression equations describing the linearity for each method.
Dosage Form Preparations (SUPRAX® 100 mg/5mL POS (60mL)&(30mL): To reconstitute, suspend SUPRAX®100 mg/5mL POS (60mL)&(30mL) with 40 mL and 20 mL water, respectively. Tap the bottle several times to loosen powder contents prior to reconstitution. Add approximately half the total amount of water for reconstitution and shake well. Add the remainder of water and shake well. Transfer 2 mL from the prepared flask into 1000mL volumetric flask and complete to the mark with diluents to obtain concentrations of CFX & SDB (40 &1μg /mL), respectively.
Construction of Calibration Curves
Different aliquots equivalent to 10-50μg /mL and 1-30 μg /mL of CFX and SDB, respectively, are separately withdrawn from their respective working standards into separate series of 10 mL volumetric flasks, and the volumes are made up to the mark with the diluent. Zero order absorption spectra of CFX and SDB are scanned in the range of 200-400 nm (Figure 2) and stored. The calibration curves relating the obtained absorbances to the corresponding concentrations are constructed and the regression equations are computed for each method.

Direct spectrophotometric method (D0)

In zero order absorption spectra, CFX is directly determined using its extended spectra at its λmax 290 nm, the calibration curve relating the obtained absorbances and the corresponding concentrations in the range 10-50 μg/mL is constructed and the regression equation is calculated (Figure 3).
First Derivative of Ratio Spectra Spectrophotometric Method (1DD)
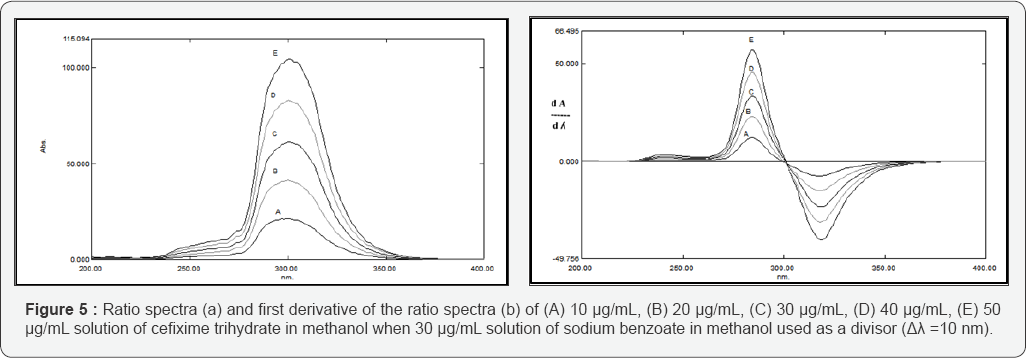
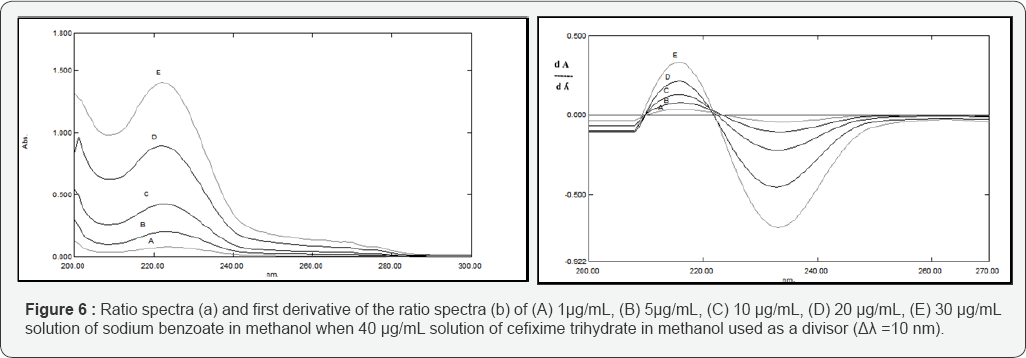
The stored spectra of the pure standard CFX are divided by the standard spectrum of 30 μg/mL of SDB and their first derivatives are obtained using Δλ = 10 and scaling factor = 10.The amplitudes of the first derivative of the ratio spectra at one maximum 284 nm and one minimum at 318 nm are plotted versus the corresponding concentrations of CFX and the calibration graph is constructed, also the stored spectra of the pure standard SDB are divided by the standard spectrum of 40 μg/mL of CFX, thereafter the first derivative is obtained using Δλ = 10 and scaling factor = 10. The amplitudes of the first derivative of the ratio spectra at one maximum 216 nm and one minimum at 234 nm are plotted versus the corresponding concentrations of CFX and the calibration graphs are constructed.
Simultaneous Ratio Subtraction Method (SRSM)
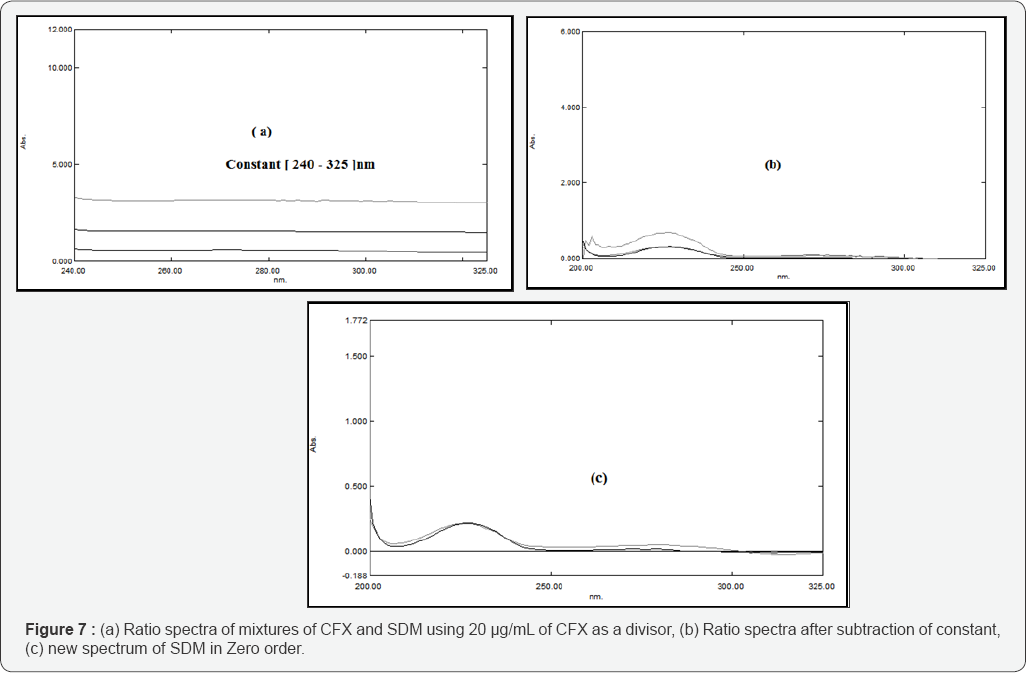
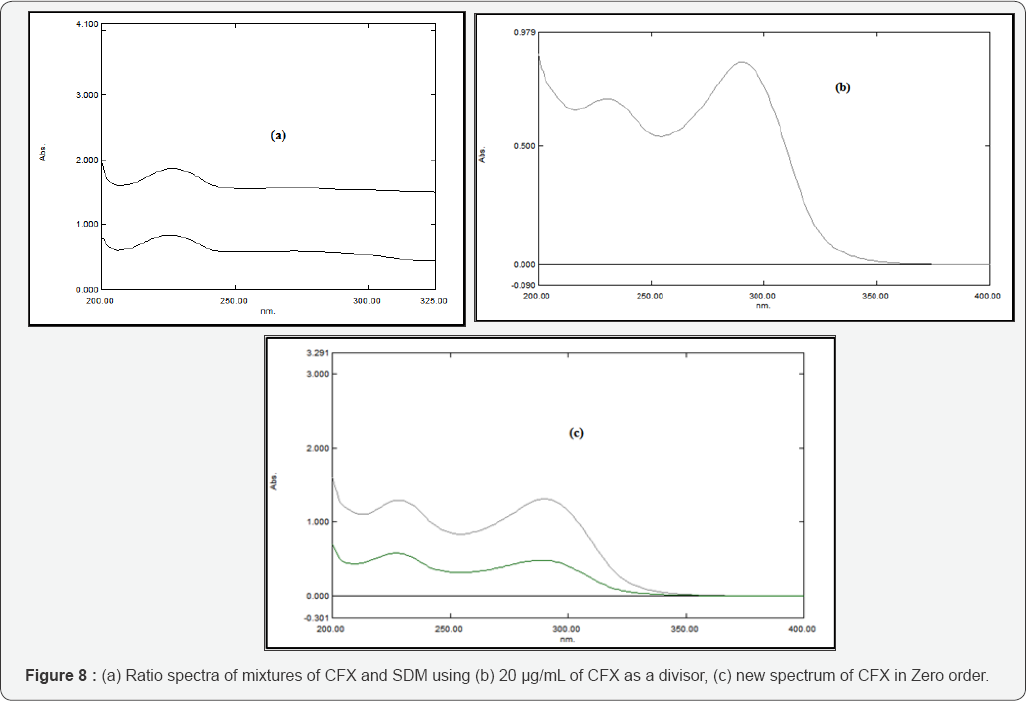
Lotfy and Hegazy introduced (SRSM) [53] for the analysis of a mixture of two drugs X and Y having overlapping spectra. Ratio subtraction method plays an important role in solving overlapped spectra between two drugs. The stored spectra of binary mixtures of CFX and SDB are divided by the absorption spectrum of CFX (20 μg/mL), then subtract the constant value at the plateau region 240-325 nm, then the zero-order absorption spectrum of SDB is obtained at 227 nm by multiplying the obtained curve after subtraction by 20 μg/mL of CFX as divisor. Calibration curves relating the absorbance of zero order spectra of SDB at 227.0 nm versus their corresponding concentrations are constructed and the regression equations are computed. For obtaining the second component (CFX), another extension of the already developed method has been established as a new approach in which CFX is determined by multiplication of 20 μg/mL of CFX divisor by the previously obtained constant, thus, the zero-order absorption spectrum of CFX is obtained at λmax equals 290 nm. Calibration curves relating the absorbances of zero order spectra of CFX against their corresponding concentrations are constructed and the regression equations are computed.
Ratio Difference Spectrophotometric Method (RDSM)
The scanned spectra of binary mixtures of CFX and SDB are divided by the absorption spectrum of CFX (40 μg/mL)and SDB (30 μg/mL), respectively. Then, the ratio spectra are recorded. Calibration curves are constructed by plotting the difference between the amplitude at two wavelengths 231and 290 nm for CFX and 223 and 250 nm for SDB versus the corresponding concentrations and the regression equations are computed.
Results and Discussion
The fundamental aim of this work is to introduce novel, simple, sensitive, accurate and precise spectrophotometric methods for the determination of binary mixture of the two drugs CFX and SDB in their pure form and pharmaceutical formulation and resolving overlapped spectra in zero order in the region 200 - 250 nm, which couldn't be resolved neither by first nor the second derivative techniques as shown in (Figure 4), so other methods are applied to overcome the overlapping as 1DD, SRSM and RDSM. For convenience, each one will be discussed separately.
Methods Development and Optimization
Selection of the solvents, divisor and wavelengths is the key factors to the adjustment and development of the method, so different solvents were tried such as water, 0.01 M HCl, 0.01 M NaOH and methanol. The use of methanol as a solvent led to the development of the most resolved spectra with minimum noise and maximum sensitivity. Also, selection of the divisor at concentrations of 20 and 40 μg/mL of CFX and 30 μg/mL of SDB in the SRSM, 1DD and RDSM which showed good selectivity and recovery. Different wavelengths were chosen to determine CFX and SDB, respectively. That of 284 nm for CFX 234 nm for SDB proved to be appropriate in the 1DD; similarly, the difference between the amplitudes at two wavelengths 231,290 nm for CFX and 223, 250 nm for SDB are most suitable in the RDSM. Finally, 227 and 290 nm for CFX and SDB, respectively gave good selectivity in the SRSM concerning laboratory prepared mixtures.
First Derivative of Ratio Spectra Spectrophotometric Method
Figure 4 shows the overlapped spectra between the binary mixture of CFX and SDB. It couldn't be resolved neither by first nor the second derivative techniques. However, the proposed method proved its capability for solving their overlapped spectra as shown in Figures 5&6, where the stored spectra of the pure standard CFX are divided by the standard spectrum of 30 μg/mL of SDB and their first derivatives are obtained using Δλ = 10 and scaling factor = 10 and peak amplitudes are measured at 284 nm versus the corresponding concentrations of CFX and the calibration graphs are constructed. Also, the stored spectra of pure standard SDB are divided by the standard spectrum of 40 μg/mL of CFX and the peak amplitudes are measured at 234 nm against the corresponding concentrations of SDB and the calibration graphs are constructed.

Simultaneous Ratio Subtraction Method
This method is applied to solve the overlapping spectra between the constituents of the binary mixture CFX & SDB by dividing the spectrum of the mixture by a known concentration of CFX (20 μg/mL) as a divisor, subtract the constant value at the plateau region 240-325 nm then multiply the obtained spectrum by the divisor 20 μg/mL of CFX. Thus, the zero-order absorption spectrum of SDB is obtained at 227 nm as shown in Figure 7. Calibration curves relating the absorbances of zero order spectra of SDB at 227.0 nm versus their corresponding concentrations are constructed and the regression equations are computed. The original zero order spectrum of CFX is obtained at 290 nm after multiplication of the constant in the laboratory prepared mixture by the CFX (the divisor), (Figure 8).Calibration curves relating the absorbance of the zero order spectra of CFX versus their corresponding concentrations are constructed and the regression equations are, similarly, computed.
Ratio Difference Spectrophotometric Method
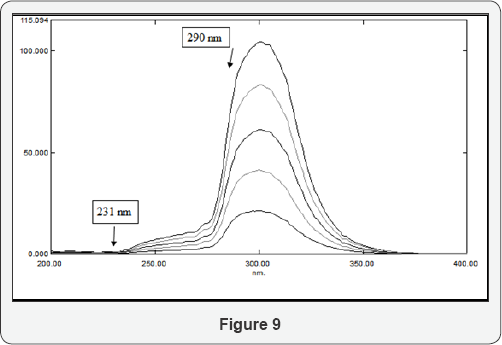
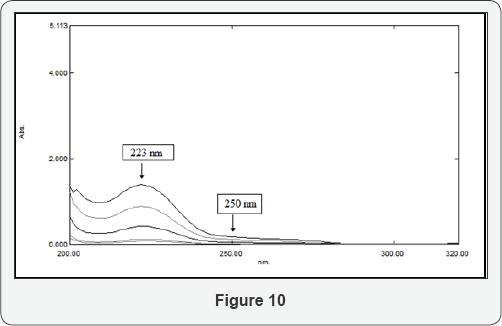
Two factors should be appropriately fulfilled during the application of RSDM, namely, the choice of the divisors and the two selected wavelengths; the selected divisor should be adjusted between the minimum noise and maximum sensitivity; the second is that each of the selected wavelengths has a contribution of the interfering substance. In the RSDM, the difference between the amplitudes at two points 223 -250 nm are selected for determination of SDB using the absorption spectrum of CFX (40 μg/mL)as a divisor, (Figure 9). Similarly, the difference between the amplitudes at two points 231& 290 nm are selected for the determination of CFX using the absorption spectrum of SDB (30 μg/mL) as a divisor, (Figure 10). Calibration curves are constructed by plotting the difference between the amplitudes at the two wavelengths 231,290 nm for CFX and 223, 250 nm for SDB versus the corresponding concentrations and the regression equations are computed.
Validation of the Analytical Method
The method was validated, in accordance with ICH guidelines (ICH Q2R1), regarding linearity, precision, accuracy, selectivity, LOD and LOQ [56].
Linearity and Range
The linearity of the proposed methods is obtained in the concentration range (10.0-50.0 μg/mL) for CFX and (1.0 -30.0 μg/mL) for SDB. Calibration curves are composed by plotting the absorbance against the corresponding concentration. The obtained results of correlation coefficients, slope and intercept indicate the good linearity. Results are shown in Table1.
Precision
Repeatability: Expresses within-laboratories variations: The intraday RSD % (n = 3), average of three concentrations (10, 20 and 30μg/mL) for each of CFX and SDB, respectively are repeated three times within the day. Good results are obtained as shown in Table 1.
Intermediate precision: The Interday RSD % (n = 3), average of three concentrations (10, 20 and 30μg/mL) for each of CFX and SDB, respectively are repeated three times in three successive days. Good results are obtained and presented in Table 1.
Detection and Quantitation limits
These approaches are based on the Standard Deviation of the Response and the Slope. A specific calibration curve should be studied using samples containing an analyte in the range of LOD and LOQ. The residual standard deviation of a regression line or the standard deviation of y-intercepts of regression lines may be used as the standard deviation. LOD=3.3*σ /slope and LOQ =10xσ /slope, where σ = the standard deviation of the response (Table 1).
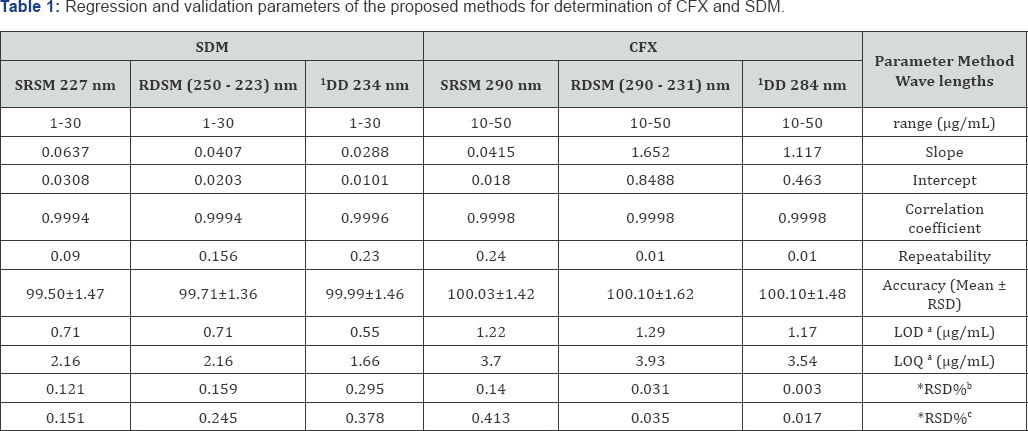
aLimit of detection (3.3* σ/Slope) and limit of quantization (10* σ /Slope).
*RSD%b&*RSD%c:the intra-day and inter-day respectively (n = 3) relative standard deviation of concentrations (10, 20, 30μg/mL).
Accuracy and recovery
Accuracy of the proposed methods is calculated as the mean percentage recoveries of pure samples of the studied drugs. Accuracy is assessed using three different concentrations (10 & 20 & 30 μg/mL) for CFX and SDB, respectively within the linearity range (i.e. three concentrations and three replicates). Concentrations are calculated from the corresponding regression equations. The mean % recoveries for CFX and SDB are between 98% and 102%. These data are shown in Table 1. Accuracy is further assessed by applying the standard addition technique to SUPRAX®100 mg/5mL POS (60mL), where good recoveries of the proposed methods are obtained, Table 2.
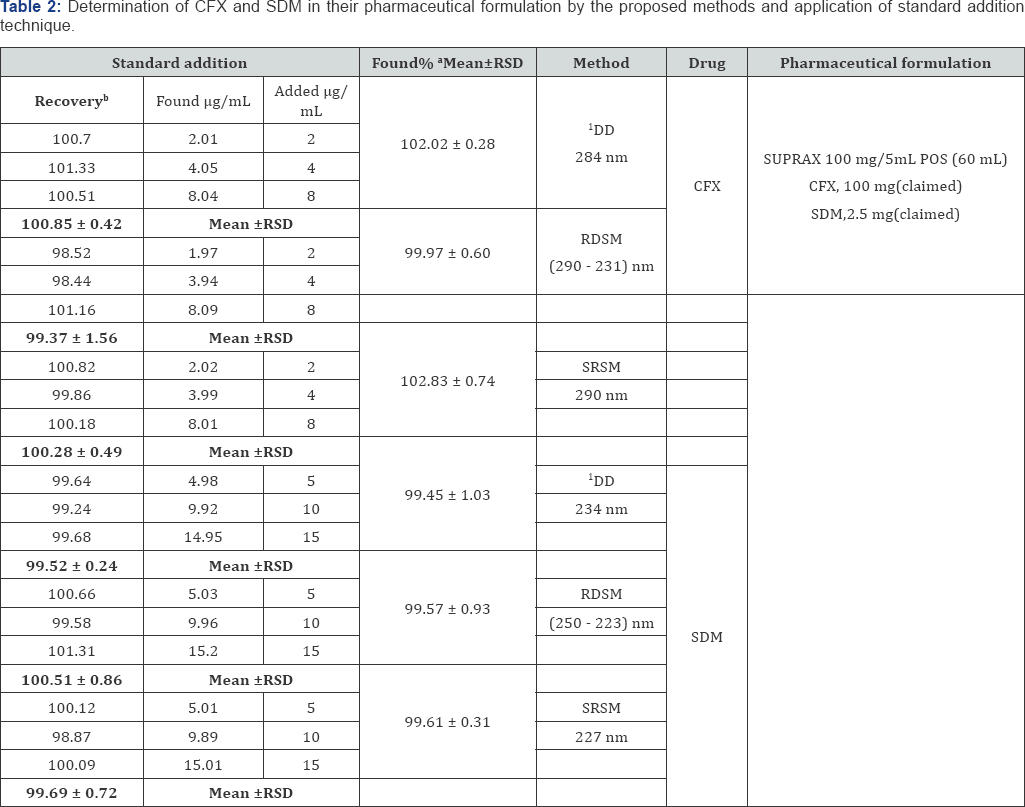
a Average of 6 determinations.
b Average of 3 determinations

*Average of three determinations
Selectivity
The selectivity of the laboratory prepared mixture containing both drugs in different ratios is determined within the linearity range. The proposed methods are assessed by analysis of laboratory prepared mixtures containing different ratios of CFX and SDB which gave good accuracy and recovery for CFX and SDB as shown in Table 3.
Statistical analysis
The results obtained for analysis of CFX and SDB by the proposed methods are statistically compared with those obtained by official [5] and the HPLC methods [55] for the simultaneous determination of both drugs in a mixture. The results showed no significant differences among the proposed spectrophotometric methods and the reference methods as presented in Table 4. In order to compare the ability of the proposed methods for the simultaneous determination of CFX and SDB, their results versus those obtained by applying the official [5] and the reported [54] methods are subjected to statistical analysis using one way ANOVA test. Results of ANOVA analysis showed that no significant differences among the proposed spectrophotometric methods and compared ones as presented in Table 5.
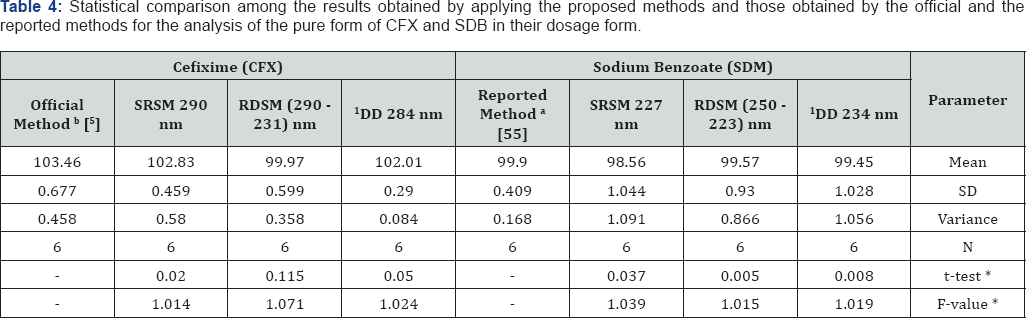
The values in parenthesis are corresponding to the theoretical values of t and F (p = 0.05).
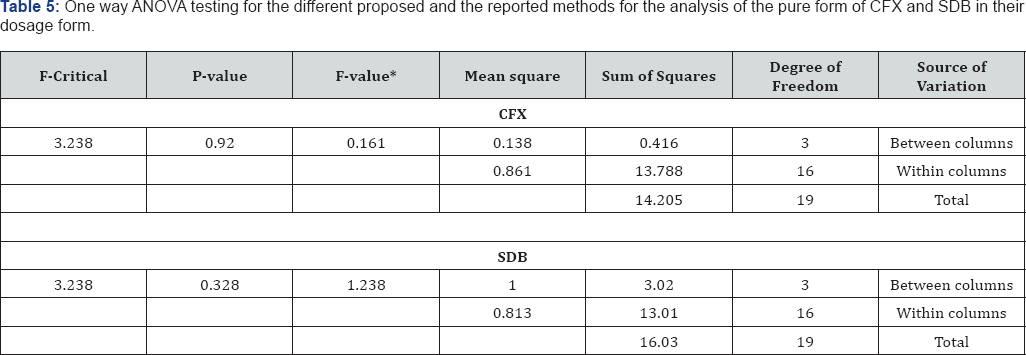
* There was no significant difference between the methods using one-way ANOVA at p < 0.05.
Conclusion
Novel, simple, precise and accurate spectrophotometric methods are performed for the simultaneous determination of binary mixture of CFX and SDB in pure and dosage form. Distinct features of the proposed methods are that being of low cost, rapid and neither require sophisticated instruments nor special software; also, could be easily applied for the routine analysis of the drugs either in their pure bulk powders and in dosage form in quality control laboratories.
Acknowledgement
The authors are thankful to the Publishers of the Global Journal of Otolaryngology for the provided publication gift.
References
- British Pharmacopoeia, Stationary Office, Medicines and Healthcare Products Regulatory Agency, London, Vol. II, 2017. European Pharmacopeia (9th edn) 2017.
- Sweetman SC, (2011) Martindale: The Complete Drug Reference (37th Edn), The Pharmaceutical Press, London.
- Raymond C Rowe, Paul J Sheskey, Marian E Quinn (2012) Handbook of Pharmaceutical Excipients 7th edn. Pharmaceutical Press, London, UK.
- The United States Pharmacopoeia, 38th Revision, NF 33, The United States Pharmacopoeia Convention Inc., June 1, 2015
- Shah V, Raj H (2012) Development and Validation of Derivative Spectroscopic Method for Simultaneous Estimation of Cefixime Trihydrate and Azithromycin Dihydrate in Combined Dosage Form. International Journal of Pharmaceutical Sciences and Research 3(3): 14-25.
- Sutar SV, Sancheti H, Patil SS (2013) Spectrophotometric Method for Cefixime Trihydrate by Using Hydrotropic Agent. International Science Press, India 6 (2):80-85.
- Maheshwari RK, Kinariwala M, Saxena M, Gahlot M, Chaki R, et al. (2008) Spectrophotometric Analysis of Cefixime Trihydrate Tablets using Metformin Hydrochloride as Hydrotropic Solubilizing Agent. Asian Journal of Chemistry 20(8): 6047-6050.
- Ethiraj T, Ramadoss R, Amudha M (2012) Sensitive spectroscopic method for content analysis of cefixime in solid dosage form using hydrotropy phenomenon. Chronicles of Young Scientists 3(4): 299303.
- Azmi SNH, Iqbal B, Al Mamari JK, Al Hattali KA, Al Hadhrami WN (2014) Method Development and Validation for the Determination of Cefixime in Pure and Commercial Dosage Forms by Specrophotometry. International Journal of Chemical, Molecular, Nuclear, Materials and Metallurgical Engineering 8(6): 595-601.
- Kumar A, Kishore L, Nair A, Kaur N (2011) Kinetic spectrophotometric method for the estimation of cefixime in pharmaceutical formulations. Der Pharma Chemica 3(4): 279-291.
- Patel SA, Patel JV (2013) Spectrophotometric method for simultaneous estimation of cefixime trihydrate and linezolid in tablet dosage form. IRJP 4(1):161-164.
- Pekamwar SS, Kalyankar TM, Tambe BV, Wadher SJ (2015) Validated UV-Visible Spectrophotometric method for simultaneous estimation of Cefixime and Moxifloxacin in Pharmaceutical Dosage Form. Journal of Applied Pharmaceutical Science 5 (01): 037-041.
- Attimarad M, Al Dhubiab BE, Alhaider IA, Nair AB, Sree HN (2012) Simultaneous determination of moxifloxacin and cefixime by first and ratio first derivative ultraviolet spectrophotometry. Chemistry Central Journal 6(1):105-112.
- Md Ahasan Ullah Nayon, Jeb Un Nesa, Md Nasir Uddin, Md Shah Amran, Umme Bushra (2013) Development and validation of UV Spectrometric Method for the Determination of Cefixime trihydrate in Bulk and Pharmaceutical Formulation. Asian Journal of Biomedical and Pharmaceutical Sciences 3(22): 1-5.
- Anees MI, Baig MS, Tathe A (2015) Determination Of Cefixime And Moxifloxacin In Pharmaceutical Dosage Form By Simultaneous Equation And Area Under Curve UV-Spectrophotometric Method. World Journal of Pharmacy and Pharmaceutical Sciences 4(3): 11721179.
- Ramadan A, Mandil H, Dahhan M (2013) UV-Vis Spectrophotometric Study for Determination of Cefixime In Pure Form and In Pharmaceuticals Through Complexation with Cu(II) Using Acetate- Noah Buffer in Water: Methanol. Int J Pharm PharmSci 5(1): 428-433.
- Shah SR, Pradhan P, Dey S (2013) Quantitative Estimation Of Cefixime And Moxifloxacin In Pharmaceutical Preparation By UV Spectrophotometric Method. International Journal of PharmTech Research 5(1): 198-204.
- Kumar R, Singh P, Singh H (2011) Development of Colorimetric Method for the Analysis of Pharmaceutical Formulation Containing both Ofloxacin and Cefixime. International Journal of Pharmacy and Pharmaceutical Sciences 3(2): 178-179.
- Khandagle KS, Gandhi SV, Deshpande PB, Kale AN, Deshmukh PR (2010) High Performance Thin Layer Chromatographic determination of Cefixime and Ofloxacin in combined tablet dosage form. Journal of Chemical and Pharmaceutical Research. 2(5): 92-96.
- Deshpande MM, Kasture VS, Gosavi SA (2010) Application of HPLC and HPTLC for the Simultaneous Determination of Cefixime Trihydrate and Ambroxol Hydrochloride in Pharmaceutical Dosage Form. Eurasian Journal of Analytical Chemistry 5(3): 227-238.
- Dhoka MV, Gawande VT, Joshi PP (2013) Validated HPTLC Method for Determination of Cefixime Trihydrate and Erdosteine in Bulk and combined Pharmaceutical Dosage Form. Eurasian J Anal Chem 8(3): 99-106.
- Rao J, Sethy K, Yadav S (2011) Validated Hptlc Method for Simultaneous Quantitation of Cefixime and Ofloxacin in Bulk Drug and in Pharmaceutical Formulation. Pharmacie Globale (IJCP) 4(05).
- Honda S, Taga A, Kakehi K, Koda S, Okamoto Y (1992) Determination of cefixime and its metabolises by high-performance capillary electrophoresis. Journal of Chromatography A 590(2): 364-368.
- Jain R, Gupta VK, Jadon N, Radhapyari K (2010) Voltammetric determination of cefixime in pharmaceuticals and biological fluids. Analytical Biochemistry 407(1): 79-88.
- Alnajjar AO (2013) Simultaneous Determination of Ofloxacin and Cefixime in Tablet Formulation using Capillary Electrophoresis. Journal of Liquid Chromatography & Related Technologies 36(19): 2687-2697.
- Bhinge SD, Malipatil SM (2016) Development and validation of stability indicating method for simultaneous estimation of cefixime and dicloxacillin using RP-HPLC method. Journal of Taibah University for Science 10(5): 734-744.
- Adam EHK, Saeed AEM, Barakat IE (2012) Development and Validation of a High-Performance Liquid Chromatography Method for Determination of Cefixime Trihydrate and its Degraded Products Formed under Stress Condition of UV Light. IJPSR 3(2): 469-473.
- Sai krishna K, Akula G, Pandey VP, Sreedevi K, Bhupathi S, et al. (2010) Validation of Reversed - Phase Hplc Method for The Estimation of Cefixime In Cefixime Oral Suspension. IJPT 2(2 ): 385-395.
- Khandagle KS, Gandhi SV, Deshpande PB, Gaikwad NV (2011) A Simple and Sensitive RP-HPLC Method for Simultaneous Estimation of Cefixime And Ofloxacin In Combined Tablet Dosage Form. International Journal of Pharmacy and Pharmaceutical Sciences 3(1): 3(1):46-84.
- Hariprasad T, Gurumoorthy P, Ali JN (2014) Analytical Method Development and Validation of Cefixime Oral Suspension by RP-HPLC as Per ICH/USP Guidelines. IJIPBART 1(1): 47-58.
- Rathinavel G, Mukherjee PB, Valarmathy J, Samueljoshua L, Ganesh M, et al. (2008) A Validated RP - HPLC Method for Simultaneous Estimation of Cefixime and Cloxacillin in Tablets. E-Journal of Chemistry 5(3): 648-651.
- Falkowski AJ, Look ZM (1987) Determination of Cefixime In Biological Samples By Reversed-Phase High-Performance Liquid Chromatography. Journal of Chromatography 422: 145-152.
- Hernandez RG, Paz LN, Mulet LS, Lopez ML, Hoogmartens J (2001) Reversed Phase High Performance Liquid Chromatographic Determination of Cefixime In Bulk Drugs. Journal of Liquid Chromatography & Related Technologies 24(15): 2315-2324.
- MengF, ChenX, Zeng Y, Zhong D (2005) Sensitive liquid chromatography- tandem mass spectrometry method for the determination of cefixime in human plasma: Application to a pharmacokinetic study. Journal of Chromatography B 819(2): 277-282.
- Talebpour Z, Pourabdollahi H, Rafati H, Abdollahpour A, Bashour Y, et al. (2013) Determination of Cefixime by a Validated Stability-Indicating HPLC Method and Identification of its Related Substances by LC-MS/ MS Studies. Sci Pharm 81(2): 493-503.
- Golcu A, Dogan B, Ozkan SA (2005) Anodic voltammetric behavior and determination of cefixime in pharmaceutical dosage forms and biological fluids. Talanta 67(4):703-712.
- Afkhami A, Felehgari FS, Madrakian T (2013) Gold nanoparticles modified carbon paste electrode as an efficient electrochemical sensor for rapid and sensitive determination of cefixime in urine and pharmaceutical samples. Electrochimica Acta 103(30): 125-133.
- Baboli MA, Varnosfaderani AM (2014) Rapid and simultaneous determination of tetracycline and cefixime antibiotics by means of gold nanoparticles-screen printed gold electrode and chemometrics tools. Measurement 47: 145-149.
- Reddy TM, Sreedhar M, Reddy SJ (2003) Voltammetric behavior of Cefixime and Cefpodoxime Proxetil and determination in pharmaceutical formulations and urine. Journal of Pharmaceutical and Biomedical Analysis 31(4): 811-818.
- Sowmya KV, Ravishankar K, Basha DP, Kiranmayi GVN (2011) Estimation of Caffeine and Sodium Benzoate in Caffeine and Sodium Benzoate Injection by Isoabsorption Method. IJPCBS 1(1): 26-31.
- Zareh MK, Mirzaei S (2004) Spectrophotometric resolution of ternary mixtures of pseudoephedrine hydrochloride, dextromethorphan hydrobromide, and sodium benzoate in syrups using wavelength selection by net analyte signals calculated with hybrid linear analysis. Analytica Chimica Acta 526(1): 83-94.
- Popović G, Čakar M, Agbaba D (2007) Simultaneous Determination of Loratadine And Preservatives in Syrups by Thin-Layer Chromatography. Acta Chromatographica 19: 161-169.
- Gören AC, Bilsel G, Simsek A, Bilsel M, Akçadağ F, et al. (2015) HPLC and LC-MS/MS methods for determination of sodium benzoate and potassium sorbate in food and beverages: Performances of local accredited laboratories via proficiency tests in Turkey. Food Chemistry 175: 273-279.
- AGçJ B, Zor SD, Dönmez OA (2016) Development and Validation of HPLC Method for the Simultaneous Determination of Five Food Additives and Caffeine in Soft Drinks. International Journal of Analytical Chemistry 2016:1-8.
- Grembecka M, Baran P, Biazewicz A, Fijaiek Z, Szefer P (2014) Simultaneous determination of aspartame, acesulfame-K, saccharin, citric acid and sodium benzoate in various food products using HPLC- CAD-UV/DAD. European Food Research and Technology 238(3): 357365.
- Can NO, Arli G, Lafci Y (2011) A novel RP-HPLC method for simultaneous determination of potassium sorbate and sodium benzoate in soft drinks using C18-bonded monolithic silica column. Journal of Separation Science 34(16-17): 2214-2222.
- Bahremand N, Eskandari S (2013) Determination of Potassium Sorbate and Sodium Benzoate in "Doogh” by HPLC and Comparison with Spectrophotometry. International Journal of Bio-Inorganic Hybrid Nanomaterials 2(3): 429-435.
- Mahboubifar M, Sobhani Z, Dehghanzadeh G, Javidnia K (2011) A Comparison between UV Spectrophotometer and High-performance Liquid Chromatography Method for the Analysis of Sodium Benzoate and Potassium Sorbate in Food Products. Food Analytical Methods 4(2):150-154.
- Harry M, Pylypiw JR, Grether MT (2000) Rapid high-performance liquid chromatography method for the analysis of sodium benzoate and potassium sorbate in foods. Journal of Chromatography A 883(1- 2):299-304.
- Khade V, Mirgane S (2014) High-performance liquid chromatography method for the analysis of sodium benzoate. International Journal of Scientific & Engineering Research 5(10):887-889.
- Ali Mb, Attia M, Bellili N, Fattouch S (2016) Development and Validation of a RP-HPLC Method for Simultaneous Determination of Betamethasone and Sodium Benzoate in Oral Liquid Pharmaceutical Formulation. Indian Journal of Pharmaceutical Sciences 78(3): 402408.
- Esfandiar Z, Badiey M, Mahmoodian P, Sarhangpour R, Yazdani E, et al. (2013) Simultaneous Determination of Sodium Benzoate, Potassium Sorbate and Natamycin Content in Iranian Yoghurt Drink (Doogh) and the Associated Risk of Their Intake through Doogh Consumption. Iranian J Publ Health 42(8): 915-920.
- Lotfy HM, Hegazy MA (2013) Simultaneous determination of some cholesterol-lowering drugs in their binary mixture by novel spectrophotometric methods. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 113: 107-114.
- Hassouna MEM, Abdelrahman MM, Mohamed MA (2017) Validation of a Novel and Sensitive RP-HPLC Method for Simultaneous Determination of Cefixime Trihydrate and Sodium Benzoate in Powder for Oral Suspension Dosage Form. J Forensic Sci & Criminal Inves 2(4):1-10.
- ICH, Q2 (R1) (2005) Validation of Analytical Procedures: Text and Methodology. ICH Harmonized Tripartite Guideline.





