Complications of Toxins and Fillers and Evidence Based Protocols for Their Immediate, Short-Term and Long-Term Management
Cleopatra Nacopoulos and Ioannis Vlastos*
University of Crete, Greece
Submission: May 01, 2017; Published: May 09, 2017
*Corresponding author: Ioannis Vlastos, University of Crete, Voreiou Ipeirou 28, Athens, Greece, Tel: 00306976141680; Email: giannisvlastos@yahoo.gr
How to cite this article: Cleopatra N, Ioannis V. Complications of Toxins and Fillers and Evidence Based Protocols for Their Immediate, Short-Term and Long-Term Management. Glob J Oto 2017; 7(4): 555718. DOI: 10.19080/GJO.2017.07.555718
Introduction
Botulium toxin (BTX) injections and fillers are an established method of cosmetic medicine. However their use is not without consequences. Their increased application in thousands of people around the world the last decade resulted in the publication of several empirical studies discussing their appropriate use, their complications and the management of their adverse events. Thus, an evidence based approach can be applied in order individual physicians to take the most informative and acceptable actions in case a complication arise in one of their patients. In other words, the aim of this minireview is the discussion of the complications of toxins and fillers through the classification of the related studies.
Complications of Toxins and their Evidence Based Management
Medical and especially aesthetic use of BTX is generally considered safe and effective. As mentioned previously, thousands of people have been treated with BTX injections with relatively few serious adverse effects. Complications of botulium toxin are related to inappropriate use (e.g. higher doses, administration in cases of contraindications, problematic product) as well as to individual reactions (e.g. patient hypersensitivity). In addition these complications can be classified as topical vs systematic or short vs long lasting.
Topical complications are by far the most common, especially those that are related to BTX main action, namely muscle paresis. Increased muscle weakness can result in an unwilling aesthetic effect or even in a more serious functional problem. Brow ptosis, upper eye-lid ptosis, lower eye-lid ptosis, lateral arching of the eyebrow, double or blurred vision, loss or difficulty in voluntary eye closure, upper lip ptosis, uneven smile, lateral lip ptosis, lower lip flattening, orbicularis oris weakness, difficulty in chewing, dysphagia, altered voice pitch and neck weakness have been reported and are the result of the paresis that BTX causes by preventing the release of the neurotransmitter acetylcholine at the neuromuscular junction. All these complications are caused when too much treatment is provided, in cases there is a defuse of the toxin to neighborhood muscles or in cases BTX is injected to a wrong place e.g. too deep injections. It obvious that appropriate injection technique is of utmost important for the prevention of these complications. Still some adverse events that are related to the diffusion of the toxin can happen. Higher concentrations have been advocated to result to a decreased spread and fewer complications.
Management of the adverse effects on muscle action: The aforementioned adverse effects on muscle action are preventable at a great degree, transient, since the action of the toxin is not permanent, and treatable in some cases. In the majority of cases, consensus recommendations have focused on the prevention of these complications and suggest no treatment when the problem is not "serious" and temporary.
- Brow ptosis and muscle dysfunctions in the area of the lower face belong in these cases [1-3] For example, inability to whistle or kiss and difficulty in chewing solid foods have been reported as case reports. However, not specific actions are proposed in review articles and watchful waiting is suggested until the nevrotoxic effect of the toxin resolves. The same applies for mild dysphagia, altered voice pitch and neck weakness that occur when the toxin may diffuse or be inadvertedly injected to affect the muscles of deglutition, the laryngeal muscles or the sternocleidomastoid.
- On the other hand, other adverse effects on muscle action may require medical intervention. For example, upper eyelid ptosis may require eye drops containing alpha- adrenergic agonists [1-4]. Apraclonidine or Phenylephrine eye drops raise the eyebrow up to 2mm by stimulating Müller muscle. Alleviation of symptoms with these drops is considered a short term management since upper eye lid ptosis may take several hours or days to fully develop and can persist up to weeks.
- In case of lower eye-lid ptosis or loss or difficulty in voluntary eye closure, lubricating eye drops or ointments and possible lid taping is required for the avoidance of exposure keratitis [1-3].
- Lateral arching of the eyebrow and 'crows feet' are examples of complications that can be alleviated by appropriate further toxin injections that can help to reduce the imbalance [5]. These kinds of treatments are based on empirical data and common sense, and since the complications are temporary and mild, no high quality randomized control trials are required for an informed and generally accepted management plan.
- Double or blurred vision is a more serious complication in terms of functionality and quality of life. Opthalmological consultation is proposed in various reviews so that the ophthalmologist can choose between various treatment options: conservative management, use of ocular occlusion, glasses with prismatic lenses or use of BTX injection into the antagonist muscle [6].
Management of the adverse effects on the injection site: These are mild and transient and no specific medical treatment is needed, e.g. application of pressure to the injection site in case of bruising may be required, or painkillers in case of headache.
Management of generalised systemic symptoms: There are standard medical protocols for allergic reactions.
Management of failures: A thorough discussion is strongly recommended with the patient prior to BTX intervention for adverse effects and the possibility of failure, e.g. due to immunoresistance.
Conclusion
Neurotoxin complications in facial aesthetics are transient and usually mild. Their management is based mostly on the accumulated experience on BTX application in thousands of patients and on experts recommendations [4,7,8].
Complications of Fillers and their Evidence Based Management
Fillers, in conjuration with toxins, comprise the most common procedures of aesthetic medicine. Several complications have been reported in the literature with the great majority of them being confined and mild. Still, there are some serious complications such as blindness. An evidence based management of the most common and serious complications are presented below.
- Inappropriate correction (over or under injection or supperficial plane of injection) can result in a poor cosmetic outcome. This is not a serious problem since newer fillers have smaller particles and can be fully resorbed. Thus, as far immediate and short-term management is of concern, hyaluranidase injection is the treatment of choice in case of inappropriate correction with hyaruronic acid. There are several case reports/series showing its effectiveness, as well as its risks [9].
- Infections can be treated with topic and/or systemic antibiotics, or even surgical drainage in case of an abscess formation. As it is happening with the majority of fillers complications, treatment options are based on previous related dermatological empirical experience and studies and there is no need (it is also unethical and impractical) for specific high quality trials on the issue. Still there are some recommendations that are based on experts opinion or descriptive studies (evidence level V or greater) and may warrant further studies. The use of valaciclovir at a dose of 2 g bid for 1 day in case of HSV reactivation can be an example of this [10].
- In contrast to infections, where immediate management is indicated, granulomas are later onset nodules and are treated with corticosteroid injections as first line treatment and antimitotic drugs, pulsed lasers and surgery as second line options. Since there are several forms of granulomas (because of the many types of fillers that are being used) and various forms of medications (as well as dosages and protocols) belonging even in the same category, eg triamcinolone or betamethasone, large control trials that can identify the most appropriate protocol (medications and dosages) for specific granulomas caused by each of the different fillers are probably scientific fiction. However, there are several studies (from case studies to control trials) of the various treatment options and medications that can be utilized for the treatment of granulomas. Repeated doses e.g. in 4 weeks cycles for the adjustment of the appropriate dose and type of medication and for the prevention of reformation is a general accepted suggestion [11].
- There are adverse events, such as bruising and small haematomas, that require no medical intervention. However, immediate actions are needed in several other cases such as sudden onset oedema and anaphylaxis. There are standard acute life support algorithms with immediate IM adrenaline for these cases, whereas less life-threatening reactions can be treated with oral steroids and antihistamines. In cases of delayed edema, short-term actions, such as oral steroids in cases of calcium hydroxyapatite fillers may be of benefit, otherwise removal of the filler is indicated [11] since currently there no safe alternatives for late foreign body reactions.
As already mentioned, even in the topic of this dissertation, some very serious complications have been reported with the use of fillers, such as blindness. This complication belong to a category of adverse events called vascular events, that range from skin ulceration and necrosis to blindness. Because new evidence suggests that vascular compromise is almost always caused by intravascular injection of filler material mimicking thrombotic or embolic ischaemic events, therapy should theoretically aim to the dislocation of the embolus into more peripheral vessels of the retinal circulation and to an increase of oxygen delivery to hypoxic tissues [12,13].
Evidence from systematic reviews of descriptive and qualitative studies (Level V) exists showing that there is no safe, feasible, and reliable treatment for iatrogenic retinal embolism. An opthalmology consultation and an MRI scan is recommended in the first instance [14,15] Current treatment options have a high failure rate. From about 100 cases with vision changes reported in the literature, only few have shown good results with the proposed treatment modalities [16]. More specifically, ocular paracentesis for decompression, vigorous ocular massage, carbogen administration, hyperbaric oxygen therapy, intraarterial fibrinolysis, although theoretically may benefit, have shown no results in the few cases that have been introduced. Intravenous administration of diuretics was effective in one case [17] as well as systemic and topical corticosteroids with IV antibiotics [18]. It seems that immediate initiation of therapy as well as multiple therapeutic steps seems to be of benefit [19]. A six step therapy (Table 1) has been proposed as being better to monotherapy, although this recommendation is based on a small series of cases [19].
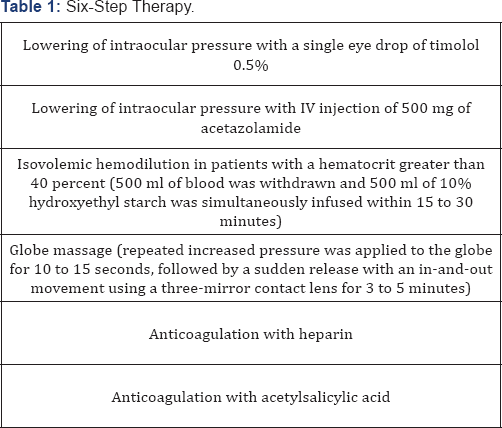
In addition, there is mostly experimental (in vitro) data showing that retrobulbar or systemic IV injection of hyaluronidase may be of benefit [20]. However this treatment is based on experts recommendations (level VII) and is effective in theory and only for hyaluronic acid. It is probably worth trying because blindness from fillers is irreversible in the great majority of cases and other options such as surgical embolectomy and transluminal neodymium: yttrium-aluminum-garnet laser embolysis are time consuming and are based in small case series showing mild if any improvement in vision [21].
References
- Niamtu J (2009) Complications in fillers and Botox. Oral Maxillofac Surg Clin North Am 21(1): 13-21.
- Dayan SH (2013) Complications from toxins and fillers in the dermatology clinic: recognition, prevention, and treatment. Facial Plast Surg Clin North Am 21(4): 663-673.
- Vartanian J, Dayan S (2005) Complications of botulinum toxin A use in facial rejuvenation. Facial Plast Surg Clin N Am 13(1): 1-10.
- Ascher B, Talarico S, Cassuto D, Escobar S, Hexsel D, et al. (2010) International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)-Part I: Upper facial wrinkles. J Eur Acad Dermatol Venereol 24(11): 1278-1284.
- Cox SE, Adigun C (2011) Complications of injectable fillers and neurotoxins. Dermatol Ther 24(6): 524-536.
- Isaac CR, Chalita MR, Pinto LD (2012) Botox® after Botox® - a new approach to treat diplopia secondary to cosmetic botulinic toxin use: case reports. Arq Bras Oftalmol 75(3): 213-124.
- Ascher B, Talarico S, Cassuto D, Escobar S, Hexsel D, et al. (2010) International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)-Part II: Wrinkles on the middle and lower face, neck and chest. J Eur Acad Dermatol Venereol 24(11): 1285-1295.
- Carruthers J, Fagien S, Matarasso SL (2004) Botox Consensus Group. Consensus recommendations on the use of botulinum toxin type a in facial aesthetics. Plast Reconstr Surg 114(6): 1S-22S.
- Cavallini M, Gazzola R, Metalla M, Vaienti L (2013) The Role of Hyaluronidase in the Treatment of Complications From Hyaluronic Acid Dermal Fillers. Aesthe Surg J 33(8): 1167-1174.
- Funt D, Pavicic T (2013) Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol 6: 295-316.
- Lemperle G, Gauthier-Hazan N (2009) Foreign Body Granulomas after All Injectable Dermal Fillers: Part 2. Treatment Options. Plast Reconstr Surg 123(6): 1864-1873.
- Lazzeri D, Agostini T, Figus M, Nardi M, Pantaloni M, et al. (2012) Blindness following cosmetic injections of the face. Plast Reconstr Surg 129(4): 995-1012.
- Park SW, Woo SJ, Park KH, Huh JW, Jung C, et al. (2012) Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol 154(4): 653-662.
- Park SW, Woo SJ, Park KH, Huh JW, Jung C, et al. (2012) Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections Am J Ophthalmol 154(4): 653-662.
- Beleznay K, Humphrey S, Carruthers JD, Carruthers A (2014) Vascular compromise from soft tissue augmentation: experience with 12 cases and recommendations for optimal outcomes. J Clin Aesthet Dermatol 7(9): 37-43.
- Beleznay K, Carruthers JD, Humphrey S, Jones D (2015) Avoiding and Treating Blindness From Fillers: A Review of the World Literature. Dermatol Surg 41(10): 1097-117.
- Peter S, Mennel S (2006) Retinal branch artery occlusion following injection of hyaluronic acid (Restylane). Clin Exp Ophthalmol 34(4): 363-364.
- Sung MS, Kim HG, Woo KI, Kim YD (2010) Ocular ischemia and ischemic oculomotor nerve palsy after vascular embolization of injectable calcium hydroxyapatite filler. Ophthal Plast Reconstr Surg 26(4): 289-291.
- Schumacher M, Schmidt D, Jurklies B, Gall C, Wanke I, et al. (2010) Central retinal artery occlusion: Local intra-arterial fibrinolysis versus con servative treatment, a multicenter randomized trial. Ophthalmology 117(7): 1367-1375.
- Carruthers JD, Fagien S, Rohrich RJ, Weinkle S, Carruthers A (2014) Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg 134(6): 1197-1201.
- Hayreh S (2007) Surgical embolus removal in retinal artery occlusion. Br J Ophthalmol 91(8): 1096-1097.





























