Bile Salts Up-Regulate MUC5AC, Not MUC5B, Mucin Expression in Cultured Airway Goblet Cell Model HT29-MTX Cell Line
Mahmoud El-Sayed Ali1, 2*, Shruti Parikh2 and Jeffrey P Pearson2
1Department of Otolaryngology, Mansoura University Hospital, Egypt
2Institute for Cell and Molecular Biosciences, Faculty of Medical Sciences, Newcastle University, UK
Submission: April 25, 2017; Published: May 04, 2017
*Corresponding author: Mahmoud El-Sayed Ali, Institute for Cell and Molecular Biosciences, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne NE2 4HH, UK, Email: msamomar@yahoo.co.uk; msali62@doctors.org.uk
How to cite this article: Ali M S, Parikh S, Pearson J P. Bile Salts Up-Regulate MUC5AC, Not MUC5B, Mucin Expression in Cultured Airway Goblet Cell Model HT29-MTX Cell Line. Glob J Oto 2017; 7(3): 555712. DOI: 10.19080/GJO.2017.07.555712
Abstract
Introduction: The production of MUC5AC and MUC5B, the major mucins expressed by airway mucosa, could be altered by various contents of laryngopharyngeal reflux. This study used the cell line HT29-MTX as an airway goblet cell model, to analyse the possible effect of bile salts on MUC5AC and MUC5B mucin production.
Methods: Goblet cell line derived from the human colon carcinoma cells HT29-MTX was cultured and challenged by exposure to various concentrations of a physiologic combination of bile salts. Modified ELISA was employed to measure of MUC5AC and MUC5B contents before and after challenge.
Results: Cultured HT29-MTX cell line maintained full viability when challenged with bile salts up to 20 ^mol/L. This stimulated MUC5AC production in a dose dependant manner and occurred within the first 24 hours after challenge. MUC5B production stayed at the base line.
Discussion: Increased MUC5AC production by the bile salt challenged HT29-MTX cell line could be due to mucin upregulation or merely due to stimulated mucin release from mucin stores in the cells. This could be achieved either directly or via the release of pro-inflammatory cytokines such as IL-8. MUC5B production may need to be studied using different cell lines. Bile salts in laryngopharyngeal reflux could alter mucin production.
Conclusion: Cultured HT29-MTX cell line can be used as a model for airway goblet cells. Bile salts challenge to these cells resulted in MUC5AC but not MUC5B release suggesting a potential rule of refluxed bile salts in modulating airway mucin expression.
Introduction
Gastric and duodenal refluxate exceeding the upper end of esophagus and reaching the hypopharynx and larynx (Laryngopharyngeal reflux) can find its way to the upper aerodigestive tract or be aspirated into lower airways. This has been reported to be associated with a wide variety of upper and lower airway disorders, including chronic pharyngitis/ laryngitis, sinusitis, secretory otitis media, isolated chronic cough, bronchial asthma, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease [1,2]. Gastric refluxate includes hydrochloric acid (HCl) and pepsin and duodenal refluxate includes bile salts and trypsin/chymotrypsin.
Tissue injury resulting from reflux can be explained by exposure to the extra-digestive mucosa to the digestive elements of reflux which are meant to break down ingested nutrients, particularly proteins. The extra digestive mucosa is susceptible to reflux injury as it normally lakes the natural defensive mechanisms/barriers to resist such an injury. The injury inflicted to the extra digestive mucosa could be direct to the cellular elements. We have shown in a previous study that the exposure of laryngeal mucosa to pepsin at a low pH can result in histological tissue damage in the form of general tissue disruption and discrete mucosal disruption [3]. Reflux injury could also be indirect by altering biological behaviour of mucosal elements.
A salient function of the upper and lower airway mucosa is mucus secretion, the biological functions of which depend mainly on mucins. Previous studies, some of which had been done in our laboratory, had shown that laryngoppharyngeal reflux can alter mucin expression [4-6] and that pepsin stimulates mucus production from goblet cells [7]. Bile salts have been found to up- regulate MUC4 expression in oesophageal cancer [8]. It had not been studied, however, whether bile salts could alter MUC5AC and/or MUC5B expression. These are the two major airway mucins produced mainly by goblet cells and secretory cells of the submucosal glands respectively [9,10]. These 2 types of cells have been found throughout the upper and lower airways and their mucin expression can be affected by various inflammatory and neoplastic conditions. Altered mucin expression and hence mucus properties can compromise mucociliary clearance and may have deleterious clinical effects [11] that could contribute in the aforementioned airway diseases.
This study investigates the potential of a physiological mixture of bile salts to stimulate mucin expression by cultured goblet cells. This was achieved by creating a physiological bile salts damage model using human goblet cells and measuring their MUC5AC and MUC5B production as makers of damage caused by exposure to bile salts.
Methods
Reagents: Unless stated otherwise reagents used were obtained from Sigma-Aldrich UK Ltd., Fisher Scientific UK Ltd. or BDH Merck Ltd.
Goblet cell culture (HT29-MTX)
A human goblet cell line derived from human colon carcinoma cells HT29 was cultured according to a previously described method [12]. HT29 cells were stepwise adapted to increasing concentrations of methotrexate (MTX), to produce a mucus-secreting phenotype. These HT29-MTX cells in culture form a homogeneous monolayer of polarized goblet cells which resemble characteristics of lung goblet cells (Figure 1), of which MUC5AC and MUC5B are the main secretory mucins.
1 ml of Dulbecco’s modified eagles medium (DMEM) supplemented with 10% foetal calf serum (FCS), 50U/ml penicillin, 50mg/ml streptomycin, 50mg/ml gentamycin, and 50|ig/ml amphotericin B (both LONZA Switzerland) was mixed into a set of liquid nitrogen frozen down cells. The supplemented medium and cells were further transferred to a T75 flask with 12 ml media each and placed in an incubator at 37oC and 10% CO2. The first change of media and PBS wash was carried out after 24 hours to prevent cell death. Afterwards, media was changed every 48 hours until cells reached confluence. Confluent cells were passaged with a trypsin solution containing EDTA. Media in the T75 flask was discarded and the confluent cells incubated with trypsin for 2 to 3 minutes until they were mobilized in the flask. The flask was then washed with several changes of culture media supplemented with 10% FCS to catch any remaining cells. The cell suspension was collected in a 25ml falcon tube and centrifuged for 7 minutes at 1100 r.p.m. The supernatant was discarded, the pellet containing the cells re-suspended with fresh media and transferred to a T75 flask for expansion followed by a 24-well plate for experimentation (0.5ml per well). For cell count cells were passaged from the T75 flask into a falcon tube with 12 ml serum-free DMEM resting media. 0.5ml of this cell suspension was mixed with 20 ml isotonic buffer and run on a cell counter.
Bile salt challenge of goblet cells
Cells were seeded at approximately 50000 per well and after reaching confluence in 24-well plates the goblet cells were rested for 24 hours by incubation with 0.75 ml serum free DMEM at pH 7.4. The cells were challenged with a physiological mix of bile salts. This was composed of 50% cholic acid, 30 % chenodeoxycholic acid, 15 % deoxycholic acid and 5 % lithocholic acid dissolved in methanol and diluted with media to give required concentrations in the proportions found in human bile [13]. We used the following concentrations: 3, 6, 9, 12, 15, 18, 20 |imol/L. Bile salts were dissolved in methanol (20 mmol/L solution), diluted down with media to the required concentrations and filtered through a sterile syringe before challenging the cells. Controls contained media free from bile salts. 0.2 ml media was collected at 24, 48 and 72 hours and assayed for MUC5AC and MUC5B by a developed ELISA as described later. Data was plotted for each bile salt concentration at 24, 48 and 72 hours.
Cell viability assay
Viability of the goblet cells after 72 hours bile salt challenge was measured with the CellTiter-Blue® Cell Viability Assay (Promega, USA) in a 96-well plate (Maxisorp, NUNC). 100% cell death was produced by the addition of 200 |il frozen methanol for approximately 5 minutes. A standard curve was produced using mixtures of live cells (LC) and methanol killed dead cells (DC) as follows: (100%DC, 75%DC/25%LC, 50%DC/50%LC, 25%DC/75%LC).
Measurement of basal mucin production
Culture media of human HT29-MTX cells were subjected to two round of Caesium Chloride equilibrium density gradient centrifugation [14] to isolate and purify polymeric mucin glycoprotein produced by HT29-MTX cells. Mucin glycoprotein content was measured by periodic acid Schiff's assay [15].
Development of indirect ELISA for mucin protein measurement
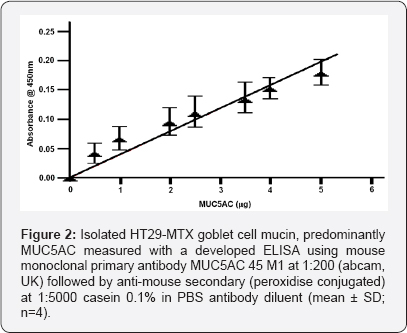
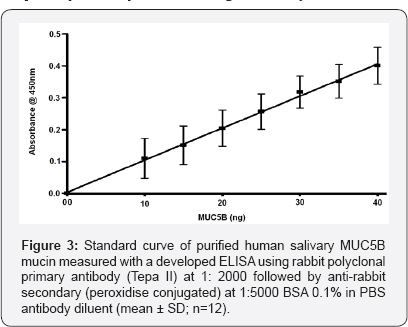
We initially used slot blot ELISA described in earlier publications [15] to measure MUC5AC and MUC5B production in culture media with pig gastric mucin and human salivary mucin as a standard for MUC5AC and MUC5B respectively. The initial MUC5AC experiments showed that MUC5AC content represent 98.8% of total basal mucin recovered from the culture media (results not shown). Due to small proportion of MUC5B produced in the culture media, the initial slot blot ELISA experiments were unsatisfactory. To improve MUC5B measurement sensitivity and reproducibility, we developed an indirect ELISA that involved the use of a 96-well plate (Maxisorp, NUNC). This also allowed a higher through-put of analysis. Furthermore, due to subsequent technical difficulties with the available MUC5AC primary antibody, we decided to use basal mucin produced by the culture media as a standard to measure the MUC5AC production by bile salt-stimulated cells. The standard MUC5AC curve was linear between 0.0 and 5.0 ng (Figure 2) which could be extended up to 10 ng after which saturation was seen and MUC5B standard curve was linear between 0.0 and 0.04 µg which could be extended up to 0.08 µg. Standard curve linearity was lost after these levels due to saturation with the antibody (results not shown).
Quantification of MUC5AC and MUC5B content by indirect ELISA
MUC5AC and MUC5B mucin collected from stimulated HT29- MTX goblet cells were measured in duplicate. 100 µl of sample (diluted 1 in 10 and 1 in 20) or standards (0-5 µg/ml basal MUC5AC or 0-0.04 ng/ml human salivary mucin) made up in PBS were coated onto a 96-well plate, covered and incubated at room temperature overnight. The plate was then aspirated, blotted dry and blocked with blocking agent (1% casein and 1% BSA in PBS for MUC5AC and MUC5B respectively) for 2 hours. Then, 100 µl of primary antibody [monoclonal MUC5AC 45 M1 (abcam, UK), diluted 1 in 200 with 0.1% casein in PBS, for MUC5AC, and polyclonal antibody (Tepa II) diluted 1 in 2000 with 0.1% BSA in PBS for MUC5B] was added. The plate was covered and incubated for 1.5 hours and aspirated, washed with three changes of 0.05% Tween 20 in PBS followed by two changes of PBS and blotted dry. 100 µl of secondary antibody [anti-mouse (peroxidise conjugated) for MUC5AC and polyclonal peroxidase conjugated anti-rabbit (Sigma A667) for MUC5B, each each diluted 1 in 5000] was then added to each well, the plate covered and incubated for 1.5 hours. The wash step was repeated and 100 µl of substrate solution 2,2'-Azino-bis 3-ethylbenzothiazoline- 6-sulfonic acid (ABTS) added, the plate covered and developed for 20 minutes and the reaction stopped with 100 |il of 1% SDS. The absorbance was measured at 405 nm (Figure 3) with a plate reader (Bio-Tek EL808). Blank controls were made by omitting the primary antibody and incubating in antibody diluent instead.
Statistical analysis
Statistical evaluations used One-Way ANOVA (Repeated Measures ANOVA, Dunn's Multiple Comparison post test) and non-parametric unpaired tests (Spearman's correlation test) with Prism (GraphPad Software Inc., La Jolla, California, USA). A p-value of 0.05 or less was considered statistically significant.
Results
The cultured HT29-MTX goblet cells remained viable through all the experiment times and there was no evidence of cell death at any of the challenge conditions. The cells produced both MUC5AC and MUC5B mucins. Basal MUC5AC was the predominant mucin compared to MUC5B by a factor of 80:1. Mean basal MUC5AC mucin secretion in the controls at all time points was 40µg/ml whereas basal MUC5B secretion was 0.5µg/ ml at all time points. MUC5AC production was stimulated by bile salts in a bile salt concentration dependant manner. Maximal MUC5AC secretion, 2 fold of basal secretion, was reached at 20 μmol/L bile salts. This basal mucin secretion levels was subtracted from the experimental bile salt stimulated levels.
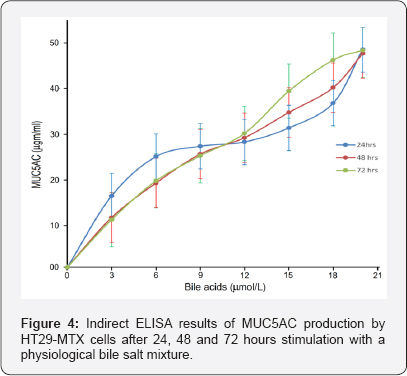
MUC5AC upregulation occurred in the first 24 hours and was not further stimulated by extended incubation (Figure 4). At the 3 time points sampled, MUC5AC concentration remained at the same level, e. g. at 20 µmol/L bile salts concentration at 24h 1ml sample contained 50 ± 9 ng (mean ± SD) mucin above the basal level, at 48h 1 ml sample contained 50 ± 5 ng (mean ± SD) and at 72h 1 ml sample contained 50 ± 5 ng (mean ± SD). MUC5AC release had occurred in the first 24 hours and no more was released after that. MUC5B secretion remained at its basal levels of 0.5 µg/ml and was not altered by incubation with tested bile salts concentrations up to 20 µmol/L (results not shown).
Discussion
This study aimed to develop a physiological damage model models to investigate the potential of bile acids to stimulate mucus production from goblet cells. Damage models had been carried out in the past, and studies have looked into the damage potential of bile salts in animal models and ex vivo [16-20]. However, there were many problems with the investigation carried out with bile salts i. e. use of conditions not pertaining to in vivo [18,19], supra-physiological bile salt concentrations which would kill the tested cells [20-22], disregard of interspecies variability in bile composition and physiology and incomparable methodology (differing sampling methods, reliability of detection & quantification methods). These factors have to be considered to avoid errors in extrapolating results of animal experiments to man. The rat for example, does not have a gallbladder and levels of lithocholic acid are almost undetectable [21].
Previous work carried out in our lab [22] has shown that exposure of primary bronchial epithelial cells to bile salt challenges of 10 ^mol/L or more was associated with significant cell injury up to cell death. However, in our current study we found that HT29-MTX cell viability was maintained at 100% when stimulated with bile salt concentrations up to 20 |imol/L. This indicates that the cell line HT29-MTX is more resistant than airway epithelial cells to bile salt challenge. This may however be a property of goblet cells compared to other epithelial cell types. The resistance could also be explained by the fact that these cells, being derived from an environment where bile and other gastric contents are common place, have an innate resistance to damage or stimulation by such components. It could also be that the HT29-MTX cells, being an immortalized human colon cancer cell line, act atypically not only in that they secrete airway specific mucins but also have higher resistance to bile acid challenge.
Lesuffleur et al. [23] have isolated MUC5AC from HT29 cell line whereas Gosalia et al. [24] have found that this cell line expresses MUC5AC at high levels with low levels of MUC5B. These studies indicate that the cell line HT29 can be used as a suitable model of airway goblet cells. We have found the same in our study with the majority of mucin produced made of MUC5AC and MUC5B expressed only at low levels. As it is difficult to grow primary lung goblet cells, we used the HT29 cell line stepwise challenged and adapted to increasing concentrations of methotrexate (HT29MTX) to obtain a homogeneous monolayer of polarized goblet cells that behaves like lung goblet cells. Therefore the HT29-MTX cells could provide an indication of the in vivo situation and act as a satisfactory goblet cell model although these cells are coming originally from colon carcinoma line and are not true lung goblet cells.
We looked at MUC5AC and MUC5B mucin production by the bile salt challenged cell line HT29-MTX as these are the 2 major mucins expressed by airway mucosa as concluded from our previous studies [14,15]. MUC5AC is an epithelial mucin mainly expressed in goblet cells of surface epithelium and to a lesser extent in the submucosal glands (SMG) whereas MUC5B is a glandular mucin mainly expressed in mucus cells of SMG and to a lesser extent in surface epithelium [9,10]. This was confirmed in our current study.
We investigated MUC5B production by this cell line to find out if this cell line behaves exclusively like goblet cells that produce mainly MUC5AC or behave also similar to the mucus secreting cells of submucosal glands which produce MUC5B as a major mucin. MUC5B production was minimal compared to MUC5AC and bile salt challenge failed to alter the MUC5B production which remained at its low basal level. This indicated that the cell line HT29-MTX behaves exclusively similar to airway goblet cells not to mucus secreting cells of submucosal glands. Further support of this comes from previous work [25] which had shown that challenging HT29-MTX cells with IL-6 and TNF-alpha did not significantly increase their production of MUC5B. However, as this is an in vitro study dealing with one type of mucus secreting airway cells, it is not known if the in vivo concomitant presence of goblet cells and SMG could result in different mucin production in response to a bile salt challenge. A co-culture cell model needs to be developed to study the expression of this mucin under more physiologic and pathologic conditions.
Mucin expression response of this cell line to bile salt challenge seems to develop and reach its maximum within the first 24 hours and plateau afterwards. Therefore, for further bile salt stimulation experiments at these concentrations it would be important to analyse the media at multiple time points up to 24 hours to obtain more clear information as regard to the temporal upregulation of MUC5AC production. Furthermore, as the increase in MUC5AC production was close to linear up to 20 |imol/L bile salts and the cells were still 100% viable in those conditions, the concentration of bile salts could be increased beyond 20 ^mol/L until viability decreases to test if and at which bile salt concentration MUC5AC production becomes maximal.
The upregulating effect of bile salts on mucin production in these cells could be due to an indirect effect of bile salts through the release of pro-inflammatory cytokines, such as tumour necrosis factor alpha, Interleukin (IL)-6 and IL-17 [26,27]. Bautista et al. [28] have shown that IL-8 regulates mucin gene expression in lung epithelial tissues in vitro, and previous work in our lab has shown that IL-8 up-regulated the secretion of MUC5AC from goblet cells in a concentration-dependent manner, with a maximum response at an IL-8 concentration of 20 ng/ ml, with the response persisting for up to 5 days [29]. It had also shown that bacterial lipopolysaccharide at concentrations up to 100 ng/ml up-regulated the expression and secretion of MUC5AC (up to 40%) and IL-8 (up to 10-fold) from HT29-MTX goblet cells in a concentration-dependant manner [30].
Given the fact that mucus hypersecretion is a key element in airway inflammation and epithelial damage [31,32] and that reflux and potential bile salt aspiration has previously been documented in paediatric and adult populations [3,33], it would be plausible to treat bile reflux as a chronic contributor to lung injury in upper airway diseases [34].
Conclusion
We looked at mucus production as marker of bile salt damage in a physiological bile salt damage model using cultured HT29-MTX cells. These cells behaved similar to airway goblet cells producing MUC5AC as the main mucin and MUC5B only as a minor mucin. MUC5AC production was stimulated within the first 24 hours, proportionate to bile slat concentrations whereas MUC5B production was not affected.
Future Areas for Research
The damage models could be widely extended in that it can be investigated whether the goblet cell line HT29-MTX produces cytokines, both constitutively and after bile salt challenge. Different individual bile salts can also be tested. Moreover, a potential uptake of bile salts into the cells could be measured, as previously shown for pepsin in laryngeal epithelial cells through receptor-mediated Endocytosis [35].
References
- Ali MS (2008) Laryngopharyngeal reflux: diagnosis and treatment of a controversial disease. Curr Opin Allergy Clin Immunol (Invited review article) 8(1): 28-33.
- Houghton LA, Lee AS, Badri H, De Vault KR, Smith JA (2016) Respiratory disease and the oesophagus: reflux, reflexes and microaspiration. Nat Rev Gastroenterol Hepatol 13(8): 445-460.
- Bulmer DM, Ali MS, Brownlee IA, Dettmar PW, Pearson JP (2010) Laryngeal mucosa; its susceptibility to damage by acid and pepsin. Laryngoscope 120(4): 777-782.
- Johnston N, Bulmer D, Gill GA, Panetti M, Ross PE, et al. (2003) Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol 112(6): 481-491.
- Samuels TL, Handler E, Syring ML, Pajewski NM, Blumin JH, et al. (2008) Mucin gene expression in human laryngeal epithelia: effect of laryngopharyngeal reflux. Ann Otol Rhinol Laryngol 117(9): 688-695.
- M S Ali, D M Bulmer, P W Dettmar, J P Pearson (2014) Mucin gene expression in reflux laryngeal mucosa: histological and in situ hybridization observations. International Journal of Otolaryngology 2014: 1-6.
- Stovold R, Corris PA, Fisher AJ, Lordan JL, Pearson JP, et al. (2008) Does pepsin in gastric juice aspirated into the lung allograft stimulate mucus hypersecretion? Journal of Heart and Lung Transplantation 27(2): S124-S.
- Mariette C, Perrais M, Leteurtre E, Jonckheere N, Hemon B, et al. (2004) Transcriptional regulation of human mucin MUC4 by bile acids in oesophageal cancer cells is promoter-dependent and involves activation of the phosphatidylinositol 3-kinase signalling pathway. Biochemical Journal 377: 701-708.
- Kettle R, Simmons J, Schindler F, Jones P, Dicker T, et al. (2010) Regulation of Neuregulin 1 beta 1-Induced MUC5AC and MUC5B Expression in Human Airway Epithelium. Am J Resp Cell Mol 42(4): 472-481.
- Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, et al. (2002) Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Resp Med 96(2): 81-86.
- Prescott E, Lange P, Vestbo J (1995) Chronic Mucus Hypersecretion in COPD and Death from Pulmonary Infection. Eur Respir J 8(8): 13331338.
- Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A (1990) Growth Adaptation to Methotrexate of Ht-29 Human Colon-Carcinoma Cells Is Associated with Their Ability to Differentiate into Columnar Absorptive and Mucus-Secreting Cells. Cancer Res 50(19): 6334-6343.
- Dewar P, King R, Johnston D (1982) Bile-Acid and Lysolecithin Concentrations in the Stomach in Patients with Duodenal-Ulcer before Operation and after Treatment by Highly Selective Vagotomy, Partial Gastrectomy, or Truncal Vagotomy and Drainage. Gut 23(7):569-77.
- Ali MS, Wilson JA, Pearson JP (2002) Nasal mucus as a model for sinus mucin gene studies in chronic sinusitis. Laryngoscope 112: 326-331.
- Ali MS, Hutton DA, Wilson JA, Pearson JP (2005) Major secretory mucin expression in chronic sinusitis. Otolaryngol Head Neck Surg 133(3): 423-428.
- Farre R, van Malenstein H, De Vos R, Geboes K, Depoortere I, et al. (2008) Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut 57(10): 1366-1374.?
- Hopwood D, Bateson MC, Milne G, Bouchier IA (1981) Effects of bile acids and hydrogen ion on the fine structure of oesophageal epithelium. Gut 22(4): 306-311.
- Lee MJ, Whitehouse MW (1965) Inhibition of Electron Transport and Coupled Phosphorylation in Liver Mitochondria by Cholanic (Bile) Acids and Their Conjugates. Biochimica et biophysica acta 100: 317328.
- Roda A, Russo C, Pasini P, Piazza F, Feroci G, et al. (1998) Antioxidant properties of bile salt micelles evaluated with different chemiluminescent assays: a possible physiological role. Journal of bioluminescence and chemiluminescence 13(6): 327-337.
- Sokol RJ, Winklhoferroob BM, Devereaux MW, Mckim JM (1995) Generation of Hydroperoxides in Isolated Rat Hepatocytes and Hepatic Mitochondria Exposed to Hydrophobic Bile-Acids. Gastroenterology 109(4): 1249-1256.
- McMaster PD (1922) Do Species Lacking a Gall Bladder Possess Its Functional Equivalent? The Journal of experimental medicine 35(2): 127-140.
- Ali Aseeri (2011) PhD thesis: Gastric aspiration, epithelial injury and chronic lung allograft rejection. Newcastle University.
- Lesuffleur T, Roche F, Hill AS, Lacasa M, Fox M, et al. (1995) Characterization of a mucin cDNA clone isolated from HT-29 mucus- secreting cells. The 3- end of MUC5AC? J Biol Chem 270L 13665-13673.
- Gosalia N, Leir SH and Harris A (2013) Coordinate Regulation of the Gel-forming Mucin Genes at Chromosome 11p15.5. J Biol Chem 288(9): 6717-6725.
- Smirnova MG, Kiselev SL, Birchall JP, Pearson JP (2001) Up-regulation of mucin secretion in HT29-MTX cells by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-6. European cytokine network 12(1): 119-125.
- Rose MC, Voynow JA (2006) Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiological reviews 86(1): 245- 278.
- Ohri SS, Vashishta A, Proctor M, Fusek M, Vetvicka V (2008) The propeptide of cathepsin D increases proliferation, invasion and metastasis of breast cancer cells. Int J Oncol 32(2): 491-498.
- Bautista MV, Chen Y, Ivanova VS, Rahimi MK, Watson AM (2009) IL-8 regulates mucin gene expression at the posttranscriptional level in lung epithelial cells. J Immunol 183(3): 2159-2166.
- Smirnova MG, Birchall JP, Pearson JP (2002) In vitro study of IL-8 and goblet cells: possible role of IL-8 in the aetiology of otitis media with effusion. Acta Otolaryngol 122(2): 146-152.
- Smirnova MG, Guo L, Birchall JP, Pearson JP (2003) LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol 221(1): 42-49.
- Hamid Q, Springall DR, Riveros-Moreno V, Chanez P, Howarth P (1993) Induction of nitric oxide synthase in asthma. Lancet 342(8886-7): 1510-1513.
- Mills PR, Davies RJ, Devalia JL (1999) Airway epithelial cells, cytokines, and pollutants. Am J Respir Crit Care Med 160(5 Pt 2): S38-43.
- Blondeau K, Dupont LJ, Mertens V, Verleden G, Malfroot A, et al. (2008) Gastro-oesophageal reflux and aspiration of gastric contents in adult patients with cystic fibrosis. Gut 57(8): 1049-1055.
- Brodzicki J, Trawinska-Bartnicka M, Korzon M (2002) Frequency, consequences and pharmacological treatment of gastroesophageal reflux in children with cystic fibrosis. Med Sci Monit 8(7): CR529-537.
- Johnston N, Dettmar PW, Bishwokarma B, Lively MO, Koufman JA (2007) Activity/stability of human pepsin: implications for reflux attributed laryngeal disease. Laryngoscope 117(6): 1036-1039.





























