Intralesional Bevacizumab in Patients with Papilomatosis Recurrent Youth Laryngia
Acosta Boett Ligia1*, Malarov Sergio3, Bastidas Yanet2, Tenías Victoria2, Rodríguez Olira3, Rodríguez Douglas3 and Acosta Yrma3
1Head of the Otorhinolaryngology Service, Children's Hospital JM de los Ríos, South America
2Adjunct Otorhinolaryngology Service, Children's Hospital JM de los Ríos, South America
3Graduate Resident of Otorhinolaryngology, Children's Hospital JM de los Ríos, South America
Submission: April 13, 2017; Published: May 04, 2017
*Corresponding author: Acosta Boett Ligia, Jefe de Servicio de Otorrinolaringología. Hospital de Niños "Dr. José Manuel de los Ríos”, Vicepresidenta de la Junta Directiva SVORL, Venezuela, South America, Email: ligiaorl2@gmail.com
How to cite this article: Acosta B L, Malarov S, Bastidas Y, Tenias V, Rodriguez O, et al. Intralesional Bevacizumab in Patients with Papilomatosis Recurrent Youth Laryngia. Glob J Oto 2017; 7(3): 555711. DOI: 10.19080/GJO.2017.07.555711
Abstract
Introduction: Human Papilloma Virus is the causative agent of Recurrent Juvenile Laryngeal Papillomatosis (PLJR), a disease characterized by benign fibroepithelial tumors in the airway. The treatment is surgical and medical with Bevacizumab (monoclonal antibody) that applied intralesional inhibits angiogenesis, reducing lesions and recurrences.
Objective: To evaluate the use of intralesional Bevacizumab in patients with recurrent juvenile laryngeal papillomatosis of the Otorhinolaryngology Service of JM de los Rios Children's Hospital.
Methodology: Exploratory, experimental, quantitative study. A sample of 17 patients between 2 and 17 years with PLR was analyzed. Epidemiological, clinical, anatomical (Nasofibrolaryngoscopy) and laboratory data were collected. The Derkay-Coltrera System (DCS) score was applied, excision of the lesions was performed by laryngeal microsurgery and Bevacizumab (Bcb) was infiltrated in 3 doses every 21 days. Data were processed using the statistical program Statgraphics Plus 5.1
Results: The male sex was the most affected in 58%, the mean age of the diagnosis was 4.4 years and the most frequent age range of 3 to 6 years. The predominant serotype was HPV type 6 in 47%, 41% was not typified, 1 patient reported 2 serotypes. 44% received 3 doses of bevacizumab, another 44% required a 4th dose and 11% required a second cycle. The DCS score decreased in 100% of the patients, 41% had a final clinical score 0, 59% had 1 point due to dysphonia, 65% had an anatomical score of 0 points. No adverse effects were seen with Bevacizumab.
Conclusion: Treatment with bevacizumabintralesional decreased relapses and is a safe medication.
Keywords: Recurrent juvenile laryngeal papillomatosis; Bevacizumab; Derkay Score
Abbreviations: PLJR: Recurrent Juvenile Laryngeal Papillomatosis; DCS: Derkay-Coltrera System; Bcb: Bevacizumab; HPV: Human Papilloma Virus; FDA: Food and Drug Administration; FIGO: International Federation of Gynecology and Obstetrics
Introduction
Recurrent juvenile laryngeal papillomatosis (PLJR) is a benign pathology caused by the Human Papilloma Virus (HPV). It is characterized by the benign proliferation of epidermoid tumors in the airway and in other areas such as the digestive tract [1,2]. The etiologic agent is HPV, a DNA virus capable of producing oncological disease belonging to the family Papovaviridae [1]. There are more than 200 different serotypes of this virus, 40 are transmitted sexually. They are divided according to their ability to produce cancer in low grade and high grade. The best known low-grade ones are 6, 11, 40, 42, 53, 54 and 57; while those of high grade are 16, 18, 31, 35, 39, 45, 51, 52, 56 and 58 [2-4]. Serotype 6 and 11 are responsible for 90% of the PLR [1,2].
The prevalence of the virus has been variable and is due to an under registration. A work done in India showed a 70% positivity in HPV 16 and 18 representing 30 to 40% of the malignancies in that country [5]. In the United States, the prevalence ranges from 4.3 per 100,000 children with a higher frequency between 2 and 4 years according to a study by Abamson [6]. In Spain a study reports 6 to 7 cases per 100,000 inhabitants evaluated in the otorhinolaryngology service [3,7]. Alarcon et al. [8], Colombia, conducted a retrospective study of 3 hospital centers, obtaining 100 patients with PLR from 2006 to 2012, aged between 2 months and 83 years, with a general average of 39 years ± 5 years, 78% of which belonged to sex male. 18 patients were children aged 8.9 +/- 4.6 years; 82 were adults aged 45.6 +/- 15.9 years. The age of diagnosis in the juvenile group was on average 6.01 +/- 4.6 years. 7% (2 pediatric patients, 5 adults) required more than 10 surgical interventions. Bello in 2001 in Venezuela typified 15 patients with PLRJ in Caracas and showed that the average age was 9 years, a minimum of 3 years and a maximum of 20 years and the serotypes found in 53% of the samples processed were 6 and 11 [9].
In 1997 Acosta et al. [10] carried out a retrospective, descriptive study in the HJM de los Ri'os in Caracas, finding 51 patients between 1990 and 1995, where 54% were female, the most frequent age group was 1 to 3 years With 47%, 4 to 6 years 29%, where 84% belonged to a low and medium low social stratum (graffar scale IV and III). In 90% of patients were obtained through vaginal delivery however the percentage of known mother with HPV 7%. With regard to surgical treatment (injury excision), 70% required less than 10 surgeries, 26% between 10 and 30, 1 patient required more than 30 surgeries during the course of the disease. Castillo H et al. [11], in 2014 performed a study in the Children's Hospital, covering all patients with laryngeal papillomatosis in our center reporting a frequency of 0.8% regarding all consultations in the otorhinolaryngology service between July 2013 and June 2014 , The mean age of diagnosis was 5.7 ± 3.2 years, the mean number of surgical interventions was 3 during the course of the disease, the most frequent viral genotype was type 6 In 50%, 11 in 11%, coinfection 6 and 11 in 11,% and the rest was non-typed. 83% of the patients were born via vaginal delivery.
Gonzalez J, in Maracaibo Venezuela, studied 34 patients with esophageal HPV, showing a male prevalence of 56.3% and a relative risk of acquiring the pathology in 4.2 with p <0.05. The age of highest prevalence was 40 to 49 years old (43.75%), the mestizo race prevailed in 75%, while 62.5% of the patients came from the Zulia state [3]. Limongu L [12], in Caracas, Venezuela studied pediatric patients with lesions in the oral cavity, finding 21 aged between 3 and 15 years old, where the female sex corresponded to 57.1%. The predominant age range was between 5 and 7 years. 9 patients reported positive typing by polymerase chain reaction for serotype 13 [12].
With respect to the clinic, there is a period of years which is asymptomatic from the infection process. The main route of infection in children is via the transvaginal route at the time of delivery with values reported in almost 90% of patients [11,12]. C-section in children may reduce the risk of papillomatosis but is not absolutely definite [13]. Smith et al. [14] in 2010 performed a prospective cohort study, with 66 pregnant women with HPV positive by polymerase chain reaction and 77 pregnant women with HPV negative. Subsequently, 418 newborns and infants were followed up for 14 months. The result obtained determined positivity per pcr in 19.7% of the children of mother HPV + and 16.9% of the children of mother HPV negative. The most frequent serotype found in children was 16. There was strong evidence among HPV + mothers and children with HPV + at 6 weeks postpartum.
The clinic is predominantly respiratory, due to progressive dysphonia, stridor, respiratory difficulty, which leads to conditioning the quality of life of the patient and categorizing it as an emergency is extreme situations [11]. The diagnosis is based on the epidemiological antecedent, the clinical picture and the finding of characteristic lesions and their subsequent study. Nasofibrolaryngoscopy plays a fundamental role in the finding of lesions. Diagnostic confirmation is through the histopathological study of the lesions obtained in a surgical procedure evidencing the presence of koilocytes, epithelial hyperplasia and hyperkeratosis determining HPV infection. There are other methods based on detection of viral DNA by polymerase chain reaction, there is currently in Europe a method capable of identifying 30 genotypes including 13 high risks. Another variant by means of a hybrid capture test, in the USA allows identifying 13 oncogenic genotypes in less than 2 hours. There is currently a rapid test in the test phase that allows detection of E6 protein in oncogenic serotypes in less than 20 minutes [14].
In 1999 Derkay et al. [15] published a score system for staging disease severity and treatment response consisting of clinical and anatomical parameters (see Annex 1). The fundamental treatment is surgery by the excision of the lesions, however there is a palliative coadjuvant treatment, one of which is Bevacizumab, a humanized monoclonal antibody produced by recombinant DNA technology in Chinese hamster ovary cells. Its trade name is Avastin (registered trademark) and its presentation is 25mg per 1 cc in vials of 4 and 16cc. It is currently approved by the US Food and Drug Administration (FDA) in adult patients for metastatic colon or rectal carcinoma; Metastatic breast cancer; Advanced unresectable, metastatic or relapsing non-small cell lung cancer associated with platinum and advanced and / or metastatic renal cell cancer in combination with interferon alpha. Also approved by the International Federation of Gynecology and Obstetrics (FIGO) in adults for advanced cancer of the epithelial ovary, fallopian tube, or primary peritoneal [1,3,16].
Appendix 1.
Derkay- Coltrera System.
Clinical Score:
- Patient's voice: normal __(0) Dysphonic __(1), Afonic___ (2) λ
- Patient’s stridor: No __(0), present with activity __(1),present at rest __(2) λ
- Urgency of surgical intervention: No __(0) Elective __(1) , Urgent __(2), Emergency __(3) λ
- Respiratory distress: No __(0), Light__ (1), Moderate__ (2) , severe __(3), Extreme __(4) λ
Clinical Total Score = __ (0-11) λ
Anatomical Score:
LARYNX:
Epiglottis: Lingual Face__ Laryngeal Face __λ
Arithenoepiglottic Replieges: Right__ Left __ λ
Vent Ventricular Bands: Right ___ Left __
Voc Vocal Strings: Right– Left –
Aritenoids: Right –Left – λ
Previous Committee – λ
Subsequent Committee λ
Subglotis λ
WINDPIPE
Trachea: ___λ
Bronquio: Derecho __Izquierdo ___λ
Tracheostomy stoma ___λ
OTHERS:
Nose —
Palate
Pharynx
Esophagus— λ
Other —Total Anatomical Score – (0-75)— λ
C. Total Score = Clinical Score + Anatomical Score (0-86)λ
Its mechanism of action is blocking angiogenesis through selective binding to vascular endothelial growth factor, a key protein for angiogenesis. Adverse effects may include allergic reactions, bleeding in different organs and systems, intestinal perforation, pulmonary thromboembolism, hypertension, rhythm disorder, thrombocytopenia, leucopenia, gastrointestinal pictures, weakness, dizziness, headache, seizures among others that are less frequently. The dose established by the FDA is for tumors and this varies according to weight and tumor, there are no pediatric doses established, however there are jobs that report doses from 5 to 7.5 in children [16]. In 2009, Zeitels et al. [17] reported that the local application of bevacizumab in laryngeal HPV lesions significantly reduces viral load by blocking angiogenesis. In 2010, Maturo S and Hartnick CJ administered injections of Bevacizumab after the excision of laryngeal papillomas with KTP laser from pediatric patients, concluding that prolonging the time between treatments and reducing them annually [18].
General Objective
To evaluate the use of intralesional Bevacizumab in patients with Recurrent Juvenile Laryngeal Papillomatosis of the Otorhinolaryngology Service of JM de los Ríos Children's Hospital.
Specific goal
- To determine the frequency according to age, sex and serotype of HPV in patients with Laryngeal Papillomatosis.
- Evaluate the evolution of patients using the Derkay- Coltrera System score.
- Identify the presence of local and / or systemic adverse effects after treatment.
Materials and Methods
a. Kind of investigation
A prospective, exploratory, experimental and quantitative study was performed.
b. Population
The population was represented by patients with Recurrent Juvenile Laryngeal Papillomatosis who attended the Otorhinolaryngology consultation of the JM de los Ríos Children's Hospital during the period between January 2014 and May 2015.
- Inclusion criteria: Age group: patients aged between 2 and 17 years with a diagnosis of PLJR who attend the J M de los Ríos Children’s Hospital located in the city of Caracas, Venezuela.
- Exclusion criteria: Patients who did not comply with the protocol, laryngeal congenital malformations.
c. Sample
For the purpose of the investigation, the type of sample used was non-probabilistic, opinionated or deliberate. It was represented by 17 patients aged between 2 and 17 years.
Methods, techniques and instruments of data collection
A standard protocol was used for data collection, personal data, age at the beginning of the study, age of diagnosis of the pathology, number of recurrences of laryngeal lesions, clinical and anatomical data of lesions based on the Derkay-Coltrera score System [15], which was applied for each surgical intervention and infiltration of Bevacizumab. Direct laryngoscopy plus excision with laryngeal microsurgery forceps of the bulky diseased tissue was performed, maintaining the mucosal covering of the vocal cords. Subsequent to previous consent of the parents, the infusion of Bevacizumab with a larynx needle was performed at a maximum of 3.0 ml at a concentration of 25 mg / 5 mL in the areas most affected by the papilloma. Before and after surgery, a complete laboratory profile was performed: complete hematology, prothrombin time, partial thromboplastin time, blood glucose, bun, creatinine, transaminases, total proteins, albumin, total and fractional bilirubin, LDH, HIV serology and VDRL.
Techniques of information analysis
The quantitative variable age was calculated descriptive statistics: the arithmetic mean ± standard error, standard deviation, minimum value, maximum and coefficient of variation. From a quantitative perspective, the variables will be analyzed from tables of simple frequency distributions (absolute and relative) and double input for the visualization of two variables simultaneously. To associate these variables we will use the non- parametric analysis of Chi square for independence between variables. Everything will be analyzed from the statistical processor Statgraphics Plus 5.1 adopting as values of statistical significance p lower than 0,05.
Results
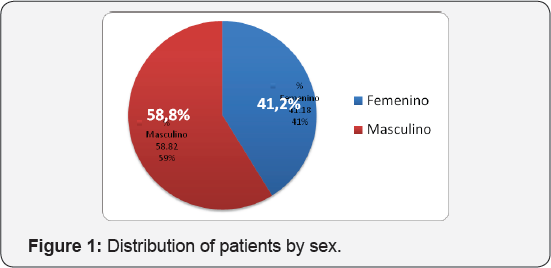
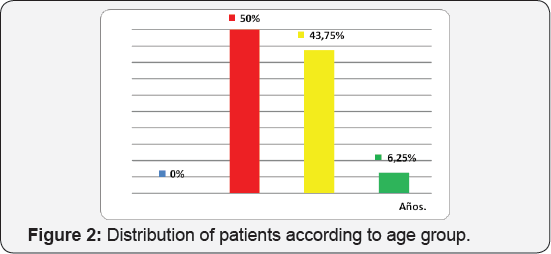
(Table 1) (Figure 1) Seventeen patients were included between 2 and 17 years of age. The male sex was represented by 10 patients, with 58.8% of the sample being studied, while the female sex represented 41.2% (7 patients) (Table 2) (Figure 2). The largest number of patients was in the range of 3 to 6 years, represented by 8 corresponding to 50% of the sample,7 patients were between 7 to 12 years old being 43, 7% and 1 patient corresponded to the range 13 to 16 being 6.25%. The male gender was higher in the range of 3 to 6 years as opposed to the 7 to 12 range where the female predominated. The mean age of diagnosis for males was 7.36 with a standard deviation of ± 4.08 years, while for females it was 7.57 with a standard deviation of ± 2.76 years (Table 3) (Figure 3).
Source: Data obtained by instrument protocol.
Source: Data obtained by instrument protocol
Source: Data obtained by instrument protocol.
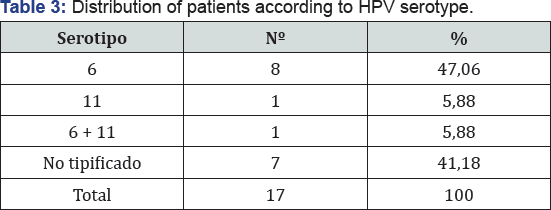
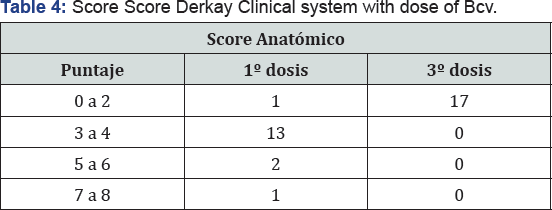
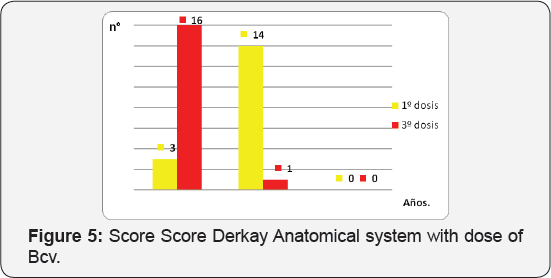
The predominant serotype was type 6 HPV in 47%, 41% was not typified (no 6, not 9, not 11), 1 patient (11%) reported positivity to 2 serotypes (6 and 11) (Table 4) (Figure 4). Regarding the clinical DCS score, 13 patients (76%) had a score between 3 and 4 initial points, 2 patients between 5 and 6 points and 1 patient was placed in 7 to 8 points before the first dose of Bcv was applied At the end of the cycle 100% (17 patients) were located with a score of 0 to 2 points, where 41% scored 0 points and 59% 1 point given for dysphonia (Table 5) (Figure 5). Regarding the anatomic DCS score, initially 16 patients, corresponding to 82% presented a score between 0 and 2, being the maximum score of 7 points in 2 patients, which presented lesions in the pharynx and esophagus. At the end of the cycle 94% was between 0 and 2 points where 65% obtained 0 point,the maximum score was determined by flat lesions that could not be removed surgically.
Source: Data obtained by instrument protocol.

Source: Data obtained by instrument protocol.
Analysis and Discussion
Regarding the distribution of PLJR in relation to sex, our study coincides with Alarcon et al in Colombia, where male sex is more frequent in pediatric ages. However, it does not coincide with the study carried out by Acosta in 1997 in our hospital where the female sex corresponded to 54% and with Bello who reported predominance of the female sex [9,10]. The mean age of our study was 7.36 for males and 7.5 years for females, being greater than that obtained by Acosta where it was 3.7 years. Alarcon reported an average of 6.01 years and Bello of 9 years [8-10]. The highest number of patients was in the range of 3 to 6 years corresponding to 50% of the sample coinciding with the works of Acosta, Alarcon and Bello. Our mean age of diagnosis for males was 7.36 with a standard deviation of ± 4.08 years, while for females it was 7.57 with a standard deviation of ± 2.76 years that there is a coincidence with the literature [1].
World literature reports serotype 6 and 11 as the cause of 90% of PLJR and this coincides with our results, however, there is a considerable percentage of non-typified patients that also coincides with other studies (Alarcon) and this is due to Diagnostic typing methods do not cover all serotypes. Regarding the patient who presented copatogenicity of 2 serotypes presented extra laryngeal lesions in the esophagus and pharynx and was the one who presented the highest score in DSC. A good outcome was observed regarding the evolution with bevacizumab, with improvement in 100% of the patients with respect to the average score and maximum score; however, 59% of the patients maintained dysphonia and 35% presented an anatomical score Due to flat lesions that could not be eliminated with the treatment. Regarding the dose administered, the literature agrees with Horcasitas [19] in an article in 2011 presenting a case of a 9-year-old female patient with PLJR for HPV 6 who underwent excision of lesion and infiltration in areas of lesions Cidofovir and bevacizumab at a dose of 15mg / 1cc with repeated doses of bevacizumab at 3 occasions every 21 days with favorable evolution, with an initial DCS index of 14 points and subsequent to the treatment of zero points without Relapses, did not report adverse effects to the drug. In our study, there was no impact on liver function or any other adverse effect in 100% of patients, demonstrating that in our study.
Bevacizumab is a safe drug and coincides with several studies. Recently Connor et al. Conducted a study with 3 groups of (Bevacizumab, cidofovir and physiological solution), infiltrating bevacizumab (Avastin) into vocal cord at doses of 0.5 cc on 4 occasions over a period of 8 weeks versus infiltration of saline and cidofovir and demonstrated that histopathologically Edema, infiltration and cellular atypia was found in the 3 groups of pigs, concluding that the infarction of Bcv, cidofovir in a single or combined form did not produce significant changes in the porcine vocal cord from the histopathological point of view and recommended studies after a higher dose (twenty) [20].
Best SR conducted a study evaluating 2 cohort studies for adult patients, a first group consisting of patients with a known diagnosis of laryngeal papillomatosis and who had received 1 single dose of Bcv of 7.5 12.5 mg, given them Infiltrated two high doses of Bcv (15 to 50 mg total), with subsequent paraclinic controls following each dose of the drug. The second group received sublesional Bcv at doses of 15 to 80mg in total without paraclinic controls. In total, 100 doses of Bcv were administered, 87 in the office and 13 in the operating room with an average of 30 mg per dose in a range of 15 to 88 mg in total. There were no local or systemic complications of Bcv in any of the 2 groups, concluding that Bcv is safe at high doses in adult patients with laryngeal papillomatosis [21].
Zeitels et al. [22] in 2009 performed a prospective study, applied pulsed KTP laser with angiolytic effect and Bcv in 5 opportunities with intervals of 4 to 6 weeks at doses of 5 to 10 mg. The results were compared with patients who only received the laser and showed that in 90% of the patients the recurrence decreased. 4 warranted another dose of Bcv between 8 and 12 weeks. No patient required surgical treatment and all had satisfactory evolvement of vocal function. Later in 2011, he carried out another prospective study with 20 adult patients with HPV lesions in both Vocal cords to which applied KTP laser with angiolytic effect in 4 opportunities with average time of 6 weeks between each application, while infiltrating bevacizumab sublesional in concentration of 7.5 to 12.5 mg in 0.3 to 0.5 cc On a string and contralaterally injected saline solution. In his study there were no local or systemic complications.
After the Bcv cycle, 3 patients had no disease, 16 had reduced lesions and only 1 patient had an increase in disease. There was improvement in all vocal quality parameters after treatment [23]. Best et al. [24,25] in 2013 studied 10 patients aged 18 months to 18 years who had more than 4 surgeries per year. Bcv was applied at 2.5 / ml concentration in 3 doses with intervals of between 2 and 3 weeks associated with pulsed KTP laser. Regarding the results, the time interval between procedures increased 5.9 weeks after infiltration (p = .002). The mean number of surgeries per year decreased as did the DCS score.
Conclusion
It was determined in our study that PLJR in a benign disease that predominates in pre-school age with a certain tendency to male gender and where serotype 6 prevails. The use of bevacizumab as a coadjuvant therapy is an alternative that provides effectiveness by reducing the values of the Derkay- Coltrera System and is a safe medication.
Recommendations
- It is suggested and proposed to carry out subsequent studies of a control case and a larger sample.
- It would be important to evaluate different dose ranges for bevacizumab.
References
- Suarez C (2010) Treatise on otolaryngology and head and neck surgery. (2nd edn).
- (2014) American Cancer Society. Cancer Facts & Figures 2014. American Cancer Society, Atlanta, USA.
- Gonzalez Jackeline (2010) Epidemiological factors associated with human papillomavirus infection in the esophagus. University of Zulia, Venezuela, South America.
- LÖrincz AT (1996) Molecular Methods for Detection of Human Papillomavirus Infection. Obstet Gynecol Clin North Am 23(3): 707715.
- Lazo P (1988) Human Papillomaviruses in Oncogenesis. Bioessay 9: 158-162.
- Abamson AL, Steimberg BM, Winkler B (1987) Laryngeal Papillomatosis: Clinical, Histopathologic and Molecular Studies. Laryngoscope 97: 687-695.
- Morgan AH, Bitsch RP (1986) Recurrent respiratory papillomatosis in children a retrospective study of management and complications. Ear Nose Throat J 65(9): 19-28.
- Alarcón H (2013) Description of socio-demographic and clinical characteristics and therapeutic outcomes in patients with recurrent respiratory papillomatosis in 3 hospitals in Bogotá. Militar University of New Granada.
- Beautiful Alford, Caibe RG (2001) Typification of human papillomavirus in juvenile recurrent laryngeal papillomatosis. Rfm [online] 24(1): 6265.
- Acosta L, Carrasquel B (1997) Laryngeal papillomatosis at J M de los Ríos Hospital, 1990 to 1995. Acta otorr venez 9(2): 43-61.
- Castillo H, Gonzalez M. Juvenile laryngeal papillomatosis and its relation to genital infection by human papillomavirus during pregnancy. Thesis. Central University of Venezuela, South America.
- Limongi Lilian (2006) Prevalence of human papillomavirus infection in the oral cavity in pediatric patients. Central University of Venezuela, South America.
- Shah K (1984) Rarity of cesarean delivery in cases of juvenile onset respiratory papillomatosis. Laryngoscope 68(6): 795-799.
- Smith E (2010) Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol 326-369.
- Concha M (2007) Diagnosis and therapy of the human papilloma virus. Rev Chil Infect 24(3): 209-214.
- Derkay CS, Malis DJ, Zalzal G, Wiatrak BJ, Kashima HK, et al. (1998) A staging system for assessing severity of disease and response to therapy in recurrent respiratory papillomatosis. Laryngoscope 108(6): 935-937
- European Medicines Agency (2014) Bevacizumab. Technical data sheet or summary of product characteristics.
- Zeitels SM, Barbu AM, Landau-Zemer T, Lopez-Guerra G, Burns JA, et al. (2011) Local injection of bevacizumab (Avastin) and angiolytic KTP laser treatment of recurrent respiratory papillomatosis of the vocal folds: a prospective study. Ann Otol Rhinol Laryngol 121(9): 367.
- Maturo SC, Hartnick CJ (2012) Juvenile-Onset Recurrent Respiratory Papillomatosis. Adv Otorhinolaryngol 73: 105-108.
- Horcasitas (2011) Manejo de la papilomatosis laríngea juvenil con tratamiento intralesional combinado de cidofovir y bevacizumab. An ORL Mex 56(3).
- Connor MP (2014) Effect of vocal fold injection of cidofovir and bevacizumab in a porcine model. JAMA Otolaryngol Head Neck Surg 140(2): 155-159.
- Best SR (2012) Safety and dosing of bevacizumab (avastin) for the treatment of recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol 121(9): 587-593.
- Zeitels SM (2009) Microlaryngoscopic and office-based injection of bevacizumab (Avastin) to enhance 532-nm pulsed KTP laser treatment of glottal papillomatosis. Ann Otol Rhinol Laryngol Suppl 201: 1-13.
- Zeitels SM (2011) Local injection of bevacizumab (Avastin) and angiolytic KTP laser treatment of recurrent respiratory papillomatosis of the vocal folds: a prospective study. Ann Otol Rhinol Laryngol 120(10): 627-634.
- Rogers DJ (2013) Use of adjuvant intralesional bevacizumab for aggressive respiratory papillomatosis in children. JAMA Otolaryngol Head Neck Surg 139(5): 496-501.





























