The Middle Ear Cleft a “Miniature Lung”: from Development to Disease
Dilesh A Mogre*
Department of ENT and Head and Neck surgery, Cenetary Hospital, India
Submission: February 15, 2017; Published: March 06, 2017
*Corresponding author: A Mogre, Speciality Medical Officer, Department of ENT and Head and Neck surgery, Cenetary Hospital, Govandi, Mumbai, India, Tel:91-9987705784;Email:damogre@gmail.com
How to cite this article: Dilesh A M. The Middle Ear Cleft a “Miniature Lung”: from Development to Disease. Glob J Oto 2017; 4(4): 555641. DOI: 10.19080/GJO.2017.04.555641
Abstract
Dysventilation in the middle ear is thought to be important in the pathogenesis of chronic otitis media and its sequelae, but there is limited understanding of cause of the ventilator disorders.The mechanics responsible for regulation of the normal middle ear pressure are similar to pulmonary physiology both being mucosa lined air filled cavities. Stalwarts like JocobSadé and Charles Bluestone were amongst the first to use the analogy. This study discusses the Pulmonary Model to explain physiology and pathology of the middle ear cleft. It is concluded that the middle ear is a very similar to the pulmonary system, and that this can be a effective teaching tool. The implications of findings in the physiology and pathophysiology of the ear are discussed.
Keywords:Middle ear cleft; Pulmonary system; Ventilation
Introduction
The middle ear cleft is part of a functional system composed of the Eustachian tube, the middle ear and the mastoid air cells posteriorly. JocobSadé in his landmark work describes the middle ear cleft as “miniature lung” which breathes through the Eustachian tube [1]. The lungs and the middle ear cleft are two mucosa lined structures of our body which need air filled cavities to function optimally.Understanding of the anatomy and physiology of the middle ear cleft by comparing it with pulmonary system in health and disease can aid the clinician in understanding the role of ventilatory dysfunction in the pathogenesis of middle ear disease.
Stark similarities between the pulmonary system and middle ear are seen during development despite dissimilar phylogeny. The pulmonary primordium and the early middle ear cleft are both derivatives of endoderm arising from endothelial duventriculi. The middle ear finds its evolution to provide a sound pressure transformer whereas, the pulmonary system is more archaic in its origin. The developing middle ear cleft divides into four ‘sacci’ or pouches which expand progressively to pneumatise the temporal bone. Middle ear folds are mesentery like interfaces between thesacci defining middle ear spaces and aeration pathways [2]. The lungsdevelop from an endodermal groove the divisions of whichbranch to form bronchi ultimately forming alveolar ‘sacculi’ [3]. Despite obvious differences in the embryological development there are marked similaritiesbetween the ‘sacci’ of developing middle ear and pulmonary ‘sacculi’.
The mucosal lining in both the middle ear and the pulmonarysystem is similar in origin and function.There is analogy between the upper airway and the tubotympanic airspace and between pulmonary parenchymaandthe atticomastoid airspace. The tubotympanic segment is lined with pseudostratified ciliated columnar epithelium similar to the “upper airway”.The most valuedcomponent of this epithelium are the cilia. The atticomastoid airspace has a monocellular epithelial layer of low cuboidal to flat cells with sparse cilia and mucous cells. The sub-mucosal connective tissue is thin with sub-mucosal vasculature and the prime function is gas exchange akin to the pulmonary parenchyma. The middle earmucosa is sensitive and reacts to changes such as infections and differences in air pressure and gas composition this mirrors mucosal behavior of pulmonary epithelium to various insults. According to Rodgers the two principal features of airway goblet cell response are rapid secretion of mucin and hyperplasia [4]. Several studies suggests that pulmonary ‘surfactant’ like substances which lower surface tension are present in the middle ear cleft and facilitate opening of the Eustachian tube [5].
Loss of air from either the lungs or the middle ear cleft has serious consequences. Even though the middle ear does not show active respiration the middle ear cleft is ventilatedintermittently by the Eustachian tube. Bluestone compares the rib cage and intercostal muscles with the tympanic membrane and handle of malleus assembly, showing movements with pressure-volume changes in the middle ear [6]. The daily ventilation of air through the middle ear is around 1-2 ml [7]. The Eustachian tube remains closed for most of the time and opens when necessary to equalize pressure (Figure 1). Akin to alveolar gas exchange, transmucosal gas exchange occurs across the middle ear cleft mucosa. Unlike previous thoughts of unidirectional gas flow, studies have shown that transmucosal gas exchange in the middle ear cleft is bidirectional.
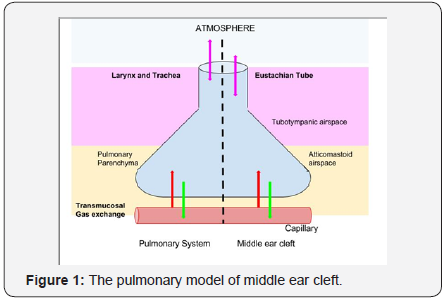
The direction of gas exchange is determined by the difference in partial pressure of gases in the middle ear cleft and blood. Similar to pulmonary receptor array the middle ear is innervated by several free nerve endings, pacinian corpuscles and other mechanoreceptors especially in the eardrum. The ear responds to atmospheric air pressure changes [8]. The pars flaccida with is unique structure plays key role as a baroreceptor in middle ear ventilation. Guild first described paraganglionic tissue near the dome of the jugular bulb and along the tympanic branch of the glossopharyngeal nerve, which resembles morphologically with the carotid body and act as chemoreceptors [9]. Studies have shown anatomic evidence of neural connections between the middle ear, brain, and Eustachian tube by which central respiratory neurons can control middle ear aeration [10]. Most of these studies have been done in lower animals and human data is lacking.
The similarities between the pulmonary system and the middle ear cleft are seen not only in health but in disease as well. Being lined by a similar epithelium the menagerie of pathogens affecting them is identical. Middle ear and the lungs are affected by viruses like Rhinovirus, Influenza virus, Parainfluenza virus, Enterovirus, Adenovirus, and Respiratory syncytial virus. Most common bacterial isolates being Haemophilus influenzae, Moraxella catarrhalis, andStreptococcus pneumonia reflecting the respiratory origins.As both the middle ear cleft and the lungs are mucosa lined air filled cavities the acute inflammatory response towards bacterial infection in acute otitis media and pneumonia follows a very similar course with stages of congestion, exudate formation, suppuration, ultimately healing or developingsequelae. The fluid transudation seen in pulmonary edema and secretory otitis media have similar origins. Some authors quote the ex vacuo theory but this theory has been challenged recently [11].
Just like luminal, mural and extramural causes of tracheobronchial obstruction by inflammation or tumorsetc. the middle ear cleft is affected by Eustachian tube obstruction. Lungs show segmental ventilation, middle ear ventilation toohas pathways and compartments. Obstruction of the tympanic isthmus, a narrow path between the tubotympanic cavity and the atticomastoid air space, is seen in middle ear dysventilation and affects air flow within the pneumatised temporal bone. Thus the epitympanic diaphragm influences partial or complete separation of the middle ear airspace [12]. The obstruction of either ventilation channel along witha tympanic membraneaffected by chronic otitis media wherein the semi-rigid collagenous layer of the eardrum is lost thus converting a physiologically stiff membrane into a flaccid one, leads to retraction pocket formation and middle ear atelectasis. This is similar to pulmonary atelectasis based on the concept of a partially collapsible gas pocket and the site of obstruction and site of atelectasis segmental, lobular or complete. Some clinical conditions affect the middle ear as much they affect the pulmonary system including laryngopharyngeal reflux, asthma and atopy.
The concepts of gaseous diffusion, pressure- volume changes are physical phenomena affecting gas lined mucosal cavities identically be it pulmonary parenchyma or the middle ear. Inferring thus, the model of the middle ear cleft based on the pulmonary system helps us to better understand the physiology and pathogenesis of middle ear dysfunction. As the pulmonary system has been studied in great detail comparing it to middle ear gives a unique perspective in educating undergraduate as well as postgraduate students about otology.
References
- Sadé J, Ar A (1997) Middle ear and auditory tube: middle ear clearance, gas exchange, and pressure regulation. Otolaryngol Head Neck Surg 116(4):499-524.
- Proctor B (1964) The development of the middle ear spaces and their surgical significance. J Laryngol Otol 78(07): 631-645.
- Kitaoka H, Burri PH, Weibel ER (1996) Development of the human fetal airway tree: analysis of the numerical density of airway endtips. Anat Rec 244(2): 207-213.
- Rogers DF (2003) The airway goblet cell. Int J Biochem Cell Biol 35(1): 1-6.
- McGuire JF (2002) Surfactant in the middle ear and eustachian tube: a review. Int J Pediatr Otorhinolaryngol 66(1): 1-5.
- Bluestone CD, Cantekin EI (1981) “How I do it” otology and neurotology. A specific issue and its solution. Management of the patulous Eustachian tube. The Laryngoscope 91(1): 149-152.
- Ingelstedt S, Jonson B (1967) Mechanisms of the gas exchange in the normal human middle ear. Actaoto-laryngologica 63(sup224): 452- 461.
- Rockley TJ, Hawke WM (1992) The middle ear as a baroreceptor. Acta Otolaryngol 112(5):816-823.
- Lewis JS, Grant RN (1951) Nonchromaffinparaganglioma of the middle ear (glomusjugulare tumor). AMA Arch Otolaryngol 53(4): 406-410.
- Eden AR, Gannon PJ, Laitman JT (1990) Mechanisms of middle ear aeration: anatomic and physiologic evidence in primates. Laryngoscope 100(1): 67-75.
- Bunne M, Falk B, Magnuson B, Hellström S (2000) Variability of Eustachian tube function: comparison of ears with retraction disease and normal middle ears. Laryngoscope 110(8): 1389-1395.
- Aimi K (1978) The tympanic isthmus: its anatomy and clinical significance. Laryngoscope 88(7 Pt 1): 1067-1081.





























