Total Trypsin and Active Trypsin: could they be used as a Biomarker for Duodenal Reflux
Shruti Parikh1, Mahmoud El-Sayed Ali1,2* and Jeffrey P Pearson1
1Institute for Cell and Molecular Biosciences, Faculty of Medical Sciences, Newcastle University, UK
2Department of Otolaryngology, Mansoura University Hospital, Egypt
Submission: January 04, 2017; Published: January 12, 2017
*Corresponding author: AMahmoud El-Sayed Ali, Institute for Cell and Molecular Biosciences, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne NE2 4HH, UK, Email:msamomar@yahoo.co.uk;msali62@doctors.org.uk
How to cite this article: Shruti P, Mahmoud El-S A, Jeffrey P P. Total Trypsin and Active Trypsin: could they be used as a Biomarker for Duodenal Reflux Glob J Oto 2017; 3(3): 555611. DOI: 10.19080/GJO.2017.03.555611
Abstract:
Introduction: Extra-gastric duodenal reflux carries proteolytic enzyme trypsin to upper aero-digestive and lower respiratory tracts and so trypsin could be a reflux biomarker. The activity of trypsin, however, can be altered by gastric acidity and pepsin. This study aimed to measure trypsin in gastric juice of reflux patients and to analyse the eligibility of trypsin as a biomarker for duodenal reflux.
Methods: Active trypsin was measured in 80 gastric juice samples, collected during gastroscopy, using a modified N-terminal assay. Total trypsin was also measured by a sandwich ELISA developed in our laboratory and compared with trypsin activity. The effect of gastric acidity and pepsin as well as bile salts on trypsin activity was studied in vitro.
Results: Active and total trypsins were detected in 80% of gastric juice samples. Active trypsin progressively and irreversibly lost its activity when incubated with pepsin at pH 2for 6 hour whereas total trypsin reactivity was reduced by only 25%. The content of active and total trypsin was not altered by incubation with bile salts at pH 7.4.
Discussion: Refluxed trypsin is likely to be partially or completely inactivated by the low stomach pH and pepsin and this could invalidate the use of active trypsin as a reflux biomarker. The sandwich Total trypsin is not significantly altered by gastric conditions and could then represent a more valid marker for duodenal reflux. The sensitivity of sandwich ELISA, however, needs to be improved to detect extra gastric trypsin.
Conclusion: Total trypsin is a more stable parameter that, with refined techniques, could be used as a biomarker for duodenal reflux.
Abbreviations: DR: Duodenal Reflux; TNBS: Trinitrobenzenesulfonic acid; SA: Succinyl Albumin; SDS: Sodium Dodecyl Sulphate; RC: Routineclinical; LTx: Lung Transplant; GJ: Gastric Juice; BALF: Broncho-Alveolar Lavage Fluid
Introduction
Duodenal reflux (DR) carries duodenal contents, including trypsin and bile salts, to the intra gastric environment. Reflux of small amounts of duodenal contents into gastric lumen can be physiologic [1] whereas excessive reflux can lead to chronic gastritis and gastric ulceration [2].Gastric reflux, containing duodenal contents can result in mucosal injury to the upper aero-digestive [3,4] and lower respiratory tracts [5,6]. Trypsin is a proteolytic enzyme which reaches its maximum proteolytic activity in an alkaline pH. Exposure of refluxed trypsin to the gastric acidity and pepsin could alter trypsin activity and subsequently reduce its injurious effect on reaching the extra gastric mucosa [7]. However, the wide use of proton pump inhibitors for treatment of reflux,by increasing gastric pH, could preserve trypsin activity and therefore would maintain its injurious effects. The detection of trypsin in the extra gastric environment could provide a solid proof of the occurrence of DR as trypsin is not normally present in the upper aero-digestive or lower respiratory tracts in higher concentrations than what can be attributed to diffusion from blood [8]. This would represent an objective non-invasive test for the occurrence of DR which would help to identify the extent it reaches in extra oesophageal areas.
It can also serve as a prognostic post-treatment parameter. The pharmacologic treatment of reflux has been based on the suppression of gastric HCl production, the sole determinant of gastric acidity necessary for pepsin activity. However, this does not reduce reflux episodes or protect from the injurious effect of other reflux components including trypsin [5] the potential damaging effect of which and the appropriate way to measure its levels in gastric juice have not been fully explored yet. The overall aim of this study was to measure and analyse trypsin contents in gastric juice of a cohort of patients with a variety of upper gastrointestinal diseases and lung transplant patients assessed for anti-reflux surgery.We measured trypsin activity by using a proteolytic activity assay and developed a sandwich ELISA for the measurement of total trypsin protein including its active and inactive forms in gastric juice. The effect of gastric environment and bile salts on trypsin activity was also studied
Materials and Methods
Ethical approval was obtained from County Durham & Tees Valley 2 Research Ethics Committee. Fasting gastric juice samples were obtained during gastroscopy from 70 patients with a variety of upper gastrointestinal diseases and 10 lung transplant patients assessed for anti-reflux surgery. Unless stated otherwise, reagents used in this study came from Sigma- Aldrich UK Ltd., Fisher Scientific UK Ltd. or BDH Merck Ltd.
Measurement of active trypsin in gastric juice
This was performed at pH 7.4 using a modified colourimetric assay [9] based on the production of new N-terminal amino groups which react with trinitrobenzenesulfonic acid (TNBS) generating trinitrophenyl derivatives that can be measured spectrophotometrically. 500 μl of 8mg/ml succinyl albumin (SA) was added to test tubes each containing 200 μl of sample or standard pig trypsin (0-2.5μg/ml) and tubes were incubated at 37oC for 30 minutes. Proteolysis was stopped by the addition of 500 μl of 4% (w/v) NaHCO3followed by 500μl of 0.05% (w/v) TNBS and 10 minutes incubation at 50oC. 500μl of 10% (w/v) sodium dodecyl sulphate (SDS) was then added followed by 500μl of 1M HCl acid. Absorbance was read at 340nm. Negative controls were made by adding the substrate and immediate addition of NaHCO3.
The effect of gastric pH (2.2 and pH 4.0) and pepsin (0.5 mg/ ml) at 37°C on trypsin (2.5 μg/ml) activity was monitored at several time points over 6 hours (extended to 7 days with pH 4.0). The effect of bile salts was also assessed, incubating 2.5μg/ ml trypsin at pH 7.4 overnight with 10 mM of a physiological bile salt mixture containing 50% cholic acid, 30 % chenodeoxycholic acid, 15 % deoxycholic acid and 5 % lithocholic acid [10] dissolved in methanol using trypsin activity at pH 7.4 as a standard in both experiments.
Development of ELISA to measure total trypsin in gastric juice
In initial experiments we used rabbit polyclonal antibody raised to bovine trypsin (Abcam ab1879) as the primary antibody. The large range of dilutions (1:8000 to 1:30000, in 0.1% BSA in PBS) suggested by the manufacturers was tested and we found that the optimal dilution 1:5000 was the best. Native trypsin has a significantly higher reactivity than heattreated trypsin (at 100oC for 5 minutes). However, trypsin levels in gastric juice obtained with this ELISA were too high (~4mg/ ml) to be physiologically possible suggesting interference in the assay. We then tried a mouse anti-trypsin monoclonal primary antibody (Abcam ab17263). However no colour development was obtained.
Development of a sandwich ELISA tomeasuretotal trypsin in gastric juice
We used the same mouse monoclonal antibody as the capture antibody bound to the ELISA plate and the rabbit polyclonal antibody (Abcam ab1879) as the detection antibody. For colour development a secondary (horseradish peroxidase conjugated polyclonal anti-goat, Sigma A6777, UK) antibody was used. The assay sensitivity was improved by increasing the concentration of detection antibody from a 1:5000 to a 1:2000 and this was the final version of sandwich ELISA used to measure total trypsin in gastric juice. 100 μl capture antibody (1:2000 dilution in 0.1% BSA in PBS) was coated onto a 96-well plate (Maxisorp, NUNC), covered and incubated at room temperature for 5 hours. The plate was then aspirated, blotted dry and blocked with 1% BSA in PBS overnight. After aspirating the plate and blotting dry, 100 μl of sample (1:25 dilution) or standards (rang 0-100 ng) made up in PBS were added to the wells and the plate was covered and incubated for 1.5 hours. The plate was then aspirated, washed three times with 0.05% Tween 20 in PBS followed by two changes of PBS and blotted dry.
Detection antibody (100 μl, 1:2000 dilutionin 0.1% BSA in PBS) was added to each well and the plate was covered and incubated for 1.5 hours. The wash step was repeated and 100 μl of secondary antibody (1:5000 dilution) was added to each well. The plate was then covered and incubated for 1.5 hours. After washing again 100 μl of substrate solution 2, 2’-Azino-bis 3-ethylbenzothiazoline-6-sulfonic acid was added to each well and the plate was covered and developed for 20 minutes. The reaction was stopped with 100 μl of 1% SDS and the absorbance measured at 405 nm with a plate reader (Bio-Tek EL808). Negative controls were made for standards and sample dilutions by omitting the detection antibody and incubating in antibody diluent instead. As previously done for the activity assay, we assessed if the reactivity of total trypsin to the antibody could be altered by gastric acidity, pepsin or bile salts. To test if long incubation times with pepsin at pH 4 could result in further loss of activity the inactivation experiment was extended up to 7 days comparing incubation at 4°C and -20°C, the temperature conditions gastric juice samples would be stored at after collection.
Results
Trypsin activity of gastric juice samples
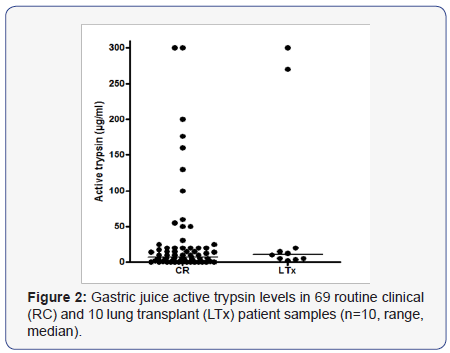
Using the N-terminal activity assay, the minimal detection level for trypsin was 0.2 μg/ml and the linear segment of trypsin standard curve extended up to 2.5μg/ml (Figure 1). One routine clinical (RC) patient’s sample was too small to be analyzed for active trypsin. Trypsin activity was detected in 55 of 69 RC and in all the lung transplant (LTx) patients. The 2 groups had a similar distribution of active trypsin levels. Median trypsin activity was 7μg/ml (range 1 - 300) and 11μg/ml (range 1 - 300) for RC and LTx patients’ samples respectively (Figure 2). The majority (52/65, 80%) of samples, had low concentrations of ≤30 μg/ml and 38% contained ≤2.5 μg/ml active trypsin. A positive correlation was found between active trypsin and pH levels (r = 0.36, p <0.001).
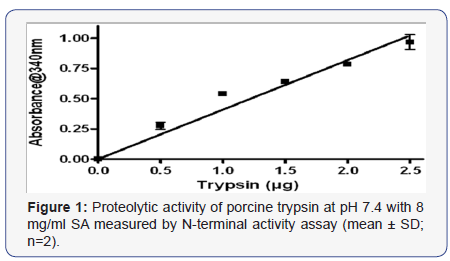
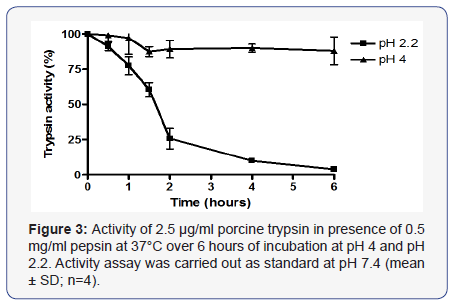
Incubating trypsin with pepsin at pH 2.2 resulted in irreversible loss of 74 +/- 7% of trypsin activity after 2 hours and complete loss of activity after 6 hours. No loss of activity was observed when incubated at pH 2.2 or pH 4.0 without pepsin. Incubation at pH 4 with pepsin caused only 12 +/- 10% loss of activity after 6 hours (Figure 3) and 20-25% after 2 days. No further loss occurred up to 7 days and no significant difference was seen for incubation at 4°C or at -20°C. Trypsin activity was not altered by overnight incubation with 10 mM of a physiological bile salt mixture at pH 7.4 (results not shown).
Analysis of total gastric juice trypsin with indirect ELISA
Bovine trypsin was tried as a standard and produced a linear standard curves for trypsin contents up to 0.21μg/ml. Comparing the reactivity of standard native bovine trypsin and heat-treated trypsin, the native trypsin showed a significantly higher assay response than the heat-treated trypsin (results not shown). Therefore native trypsin was used for further experiments. With the developed sandwich ELISA, the minimum detection level was 20 ng (0.02 μg) contained in 100μl, which correspond to 200 ng/ ml of tested samples. The standard curve was linear between 0 and 2.0 μg total trypsin above which the curve deviated from linearity and demonstrated saturation (results not shown).
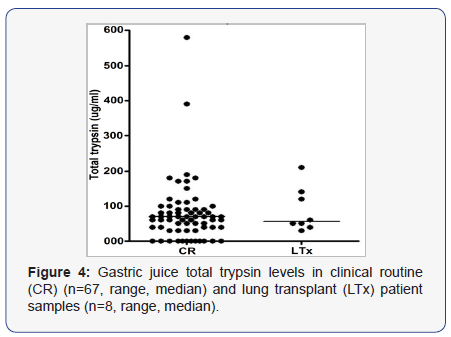
Three RC and 2 LTx patients had insufficient samples to be analysed for total trypsin leaving a total of 75 samples tested for total trypsin by sandwich ELISA. Total trypsin was detected in 63 samples (55/67 CR samples, range 30 - 580, median of 70 μg/ml and 8/8 LTx, range 40 - 210, median of 60 μg/ml) (Figure 4). Active trypsin was positively correlated (r = 0.4877, p < 0.0001) with total trypsin contents. In contrast to active trypsin, pepsin at pH 2.2 and 37oC reduced total trypsin reactivity by a maximum of 25% after 2 h and the reactivity remained stable in the following 4 hours (Figure 5). Incubation with physiologic mixture of bile salts at pH 7.4 did not affect trypsin reactivity (results not shown).

Discussion
This study aimed to assess trypsin as a biomarker for duodenal reflux by a cross-sectional analysisof trypsin contents in gastric juice (GJ) of reflux patients. Applying the modified proteolytic activity assay we obtained active trypsin content in gastric juice of reflux patients comparable with figures obtained from previous studies on pancreatic trypsin [11] and duodenal juices [12]. These studies used radioimmunoassays for trypsin measurement, in contrast to our study which used spectrophotometry and ELISA.
Trypsin activity was positively correlated with the gastric pH; the lower the pH is than trypsin’s optimum of pH 7.4 the lower the trypsin activity suggesting that low pH inactivates/ denatures trypsin. The majority of samples contained low levels of active trypsin and samples with no trypsin activity had an acidic pH level of ≤3. These observations could be explained by the inhibitory effect of pepsin and/or low gastric pH. We noted a progressive inhibition of trypsin activity up to complete loss after 6 hours when pepsin was added at a low pH. Murthy et al. [13] have noted that acidic pH denatures trypsin and the lower the pH the faster the rate of trypsin denaturation. This suggests that the inhibitory effect of gastric/duodenal acidity on trypsin activity could be indirect by altering pepsin activity.
It is necessary to take into account the attenuating effect of gastric pepsin and pH on trypsin activity when looking at trypsin as a potential reflux biomarker. Samples could be stored at pH ≥4 and the measured trypsin activity multiplied by 1.2 to correct for the effect of pH 4 and average 2 hour storage and other correction factors can be suggested according to the storage pH and duration. However, this information is difficult to obtain practically, and calculations could be incorrect and it can be concluded that active trypsin alone would be a less suitable reflux biomarker.
The mechanism and degree of mucosal injury induced by refluxed trypsin on the upper digestive and airway mucosa, and the interaction with other reflux constituents, needs to be studied. Some animal model studies have reported a synergistic damaging effect between gastric and duodenal reflux in on oesophageal mucosa [14,15], whereas other studies have postulated a protective effect of gastric juice against the development of adenocarcinoma in oesophageal mucosa exposed to duodenal contents [16]. This could, at least in part, be due to reduced trypsin activity by the action of gastric pepsin and acidity. In addition, the widespread use of PPI to treat reflux could have a detrimental effect protecting trypsin and preserving its activityby increasing gastric pH allowing a more mucosal damaging effect of trypsin whilst reducing that of pepsin [17,18].
In this study, bile acids did not alter trypsin activity and in a previous study, we found that bile acids did not alter pepsin activity [19]. This suggests that the presence of bile acids beside trypsin in the duodenal reflux does not directly or indirectly alter trypsin activity. Previous in vitro studies have suggested an enhancing effect of bile acids on trypsin proteolytic activity at neutral pH [20]. The different results could be due to different methodology as we used a different protein substrate and we incubated bile acids with trypsin alone. Bile acids and trypsin, however may augment mucosal damaging effect of each other and this requires further studies. We therefore postulated that total trypsin protein, including its active and inactive forms, could be studied as a gastroduodenal reflux biomarker and an ELISA for this could represent a suitable method for measuring trypsin levels. As a proteolytic enzyme, trypsin could digest the primary antibody used in the ELISA. This was ruled out by comparing the antibody reactivity of native and heat treated trypsin. We found that native trypsin had a significantly higher reactivity to the binding antibody compared to heat treated trypsin.
This could possibly be because coating trypsin onto the plate did not allow the enzyme active sites to digest the antibody or because the antibody was binding to trypsin at a site away from the active site of the enzyme. Heating trypsin decreased trypsin reactivity possible by modifying or destroying trypsin epitopes. In the initial ELISA the values we obtained for total trypsin were higher than those previously reported for duodenal and pancreatic trypsin [11,12] and the levels we obtained were 250- fold higher than the median levels for active trypsin. These high results could be due to cross reactivity of the primary antibody to other components of gastric juice with structural similarity to trypsin such as chymotrypsin. These results were considered physiologically impossible and were therefore rejected. We then decided to try a sandwich ELISA which reduces nonspecific binding of other components in the test sample and allows leaving the protein epitopes of the test sample exposed to bind with the other 2 primary (capture and detection) antibodies.
The mouse monoclonal antibody was used as a capture antibody and capture and detection antibodies were specific to human trypsin. In this cross-sectional study, both active and total trypsins were detected in ~80% of GJ samples and only ~20% of samples would be negative for this marker. This agrees with previous studies which have shown that DGR is common in healthy individuals [21,22].The high incidence of presence of trypsin in GJ suggest that DGR could be physiological possibly occurring along with gastric reflux. However, it is not possible from these data to draw a line between physiological and pathological DGR. Total trypsin measured by sandwich ELISA could be employed as a reliable alternative biomarker of DR with detection sensitivity of 0.2 μg/ml. However, this is unlikely to be adequate to detect refluxed trypsin in the oesophagus.
Considering the known levels of total bile acids and pepsin in the oesophagus [23,24] compared to those in the stomach [25,26], refluxed GJ into the oesophagus could be diluted ~1000 times. Extrapolating this to trypsin level in GJ found in our study, we would expect trypsin levels in the oesophagus to be 0 - 70 ng/ml which is below the minimal detection level of total trypsin measured by sandwich ELISA. This applies also to trypsin in the broncho-alveolar lavage fluid (BALF) that would be much lower given further dilution made during the BALF collection. To improve the sensitivity of sandwich ELISA, more specific monoclonal antibodies would need to be developed. As trypsin reactivity was not significantly lost by pepsin, its measurement in GJ along with the active trypsin could give more in-depth information about the load of refluxed duodenal trypsin and the impact of gastric environment that would reflect the level of gastric pepsin and gastric pH. The co-assessment of the various reflux biomarkers, including both total and active trypsin and other duodenal contents, rather than depending on a single biomarker could be the ideal approach to diagnose, evaluate and manage duodenal reflux [27].
Conclusion
Total and active trypsin can be utilized as biomarker of DR reflecting total load of refluxed trypsin and the attenuating effect of gastric pH and pepsin. This study demonstrates that trypsin could survive in gastric juice, particularly if the pH is higher than 2.2. When the gastric juice is then refluxed into the oesophagus/ upper airways and the pH is elevated into the range for trypsin activity, tissue damage could occur, particularly if it has been present in the stomach for less than 2 hours. The sensitivity and specificity of the existing detection methods for trypsin need to be improved and standard collection methods have to be established in order to obtain comparable results. In order to develop a non-invasive test trypsin levels could be measured in sputum/saliva.
References
- Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, et al. (1995) Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy reemphasized. Ann Surg 222(4): 525-531.
- Kono K, Takahashi A, Sugai H, Iizuka H, Fujii H (2006) Trypsin activity and bile acid concentrations in the esophagus after distal gastrectomy. Dig Dis Sci 51(6): 1159-1164.
- Björkman EV, Edebo A, Oltean M, Casselbrant A (2013) Esophageal barrier function and tight junction expression in healthy subjects and patients with gastroesophageal reflux disease: functionality of esophageal mucosa exposed to bile salt and trypsin in vitro. Scand J Gastroenterol 48(10): 1118-1126.
- Sifrim D (2013) Management of Bile Reflux. Gastroenterol Hepatol 9(3): 179-180.
- Blondeau K, Mertens V, Vanaudenaerde BA, Verleden GM, Van Raemdonck DE, et al. (2008) Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J 31(4): 707-713.
- Griffin SM, Robertson AG, Bredenoord AJ, Brownlee IA, Stovold R, et al. (2013) Aspiration and allograft injury secondary to gastroesophageal reflux occur in the immediate post-lung transplantation period (prospective clinical trial). Ann Surg 258(5): 705-711.
- Gotley DC, Morgan AP, Ball D, Owen RW, Cooper MJ (1991) Composition of gastro-oesophageal refluxate. Gut 32(10): 1093-1099.
- Tasker A, Dettmar PW, Panetti M, Koufman JA, Birchall JP, et al. (2002) Is gastric reflux a cause of otitis media with effusion in children? Laryngoscope 112(11): 1930-1934.
- Hutton DA, Allen A, Pearson JP, Ward R, Venables CW (1986) Separation of Pepsins in Human Gastric-Juice - Analysis of Proteolytic and Mucolytic Activity. Biochemical Society Transactions 14(4): 735-736
- Ganong WF (1989) Composition of Bile. In: Ganong WF (Ed.), Review of Medical Physiology. (14 edn): Appleton & Lange, pp. 425.
- Fedail SS, Harvey RF, Salmon PR, Brown P, Read AE (1979) Trypsin and Lactoferrin Levels in Pure Pancreatic-Juice in Patients with Pancreatic Disease. Gut 20(11): 983-986.
- Lakebakaar G, Mckavanagh S, Rubio CE, Epstein O, Summerfield JA (1980) Measurement of Trypsin in Duodenal Juice by Radioimmunoassay. Gut 21(5): 402-407.
- Murthy SN, Kostman J, Dinoso VP (1980) Effect of pH, substrate, and temperature on tryptic activity of duodenal samples. Dig Dis Sci 25(4): 289- 294.
- Narbona-Arnau B, Argente-Navarro P, Lloris-Carsi JM, Calvo-Bermudez A, Cejalvo-Lapena D (1994) Experimental endobrachyesophagus in dogs: a model without mucosectomy. Dis Esophagus 7: 112-117.
- Attwood SE, Smyrk TC, DeMeester TR, Mirvish SS, Stein HJ, et al. (1992) Duodenoesophageal reflux and the development of esophageal adenocarcinoma in rats. Surgery 111: 503-510.
- Ireland AP, Peters JH, Smyrk TC, DeMeester TR, Clark GW, et al. (1996) Gastric juice protects against the development of esophageal adenocarcinoma in the rat. Ann Surg 224(3): 358-370.
- Wilson P, Jamieson JR, Hinder RA, Anselmino M, Perdikis G, et al. (1995) Pathologic duodenogastric reflux associated with persistence of symptoms after cholecystectomy. Surgery 117(4): 421-428.
- Adhami T, Goldblum JR, Richter JE, Vaezi MF (2004) The role of gastric and duodenal agents in laryngeal injury: an experimental canine model. Am J Gastroenterol 99(11): 2098-2106.
- Ali MS, Parikh S, Chater P, Pearson JP (2012) Bile acids in laryngopharyngeal refluxate - will they enhance or attenuate the action of pepsin? Laryngoscope 123(2): 434-439.
- Gass J, Vora H, Hofmann AF, Gray GM, Khosla C (2007) Enhancement of dietary protein digestion by conjugated bile acids. Gastroenterology 133(1): 16-23.
- Schindlbeck NE, Heinrich C, Stellaard F, Paumgartner G, Mullerlissner SA (1987) Healthy Controls Have as Much Bile Reflux as Gastric-Ulcer Patients. Gut 28(12): 1577-1583.
- King PM, Pryde A, Heading RC (1987) Transpyloric Fluid Movement and Antroduodenal Motility in Patients with Gastroesophageal Reflux. Gut 28(5): 545-548.
- Johnsson F, Joelsson B, Floren CH, Nilsson A (1988) Bile-Salts in the Esophagus of Patients with Esophagitis. Scandinavian journal of gastroenterology 23(6): 712-716.
- Knight J, Lively MO, Johnston N, Dettmar PW, Koufman JA (2005) Sensitive pepsin immunoassay for detection of laryngopharyngeal reflux. Laryngoscope 115(8): 1473-1478.
- JP Pearson, S Parikh, AGN Robertson, R Stovold, IA Brownlee (2010) ‘Pepsins’ In: Effects, Diagnosis and Management of Extra-Esophageal Reflux. N Johnston, RJ Toohill (Eds), Nova Science Publishers, Inc. 4: 29-41.
- Parikh S, Brownlee IA, Robertson AG, Manning NT, Johnson GE, et al. (2013) Are the enzymatic methods currently being used to measure bronchoalveolar lavage bile salt levels fit for purpose? J Heart Lung Transplant 32(4): 418-423.
- Kia L, Pandolfino JE, Kahrilas PJ (2015) Biomarkers of Reflux Disease. Clin Gastroenterol Hepatol pii: S1542-3565(15): 1300-1302





























