The Clinical Enigma of a Midfacial Destructive Lesion
Vinay S Bhat*, Ravishankar S N, Nitha, SooryaRao and Kiran M Naik
Department of ENT, Adichunchanagiri Institute of Medical Sciences, India
Submission: September 12, 2016; Published: October 04, 2016
*Corresponding author: Vinay S Bhat, Department of ENT, Adichunchanagiri Institute of Medical Sciences, B.G Nagara, Mandya District, Karnataka, India 571448, Tel: 8495945704; Email: drvinaybhat@gmail.com
How to cite this article: Vinay S B, Ravishankar S N, Nitha, SooryaR, Kiran M N. The Clinical Enigma of a Midfacial Destructive Lesion. Glob J Otolaryngol. 2016; 2(1): 555580. DOI: 10.19080/GJO.2016.02.555580
Abstract
Midfacial destruction is a rare presentation of various neoplastic, autoimmune and infective disorders. Here we present a rare case of a midfacial distructive lesion in a young patient with xerodermapigmentosum, which was diagnosed as acantholytic squamous cell carcinoma on histopathological examination.
Keywords: Xerodermapigmentosum; Acantholytic squamous cell carcinoma; Midafacialdistructive lesion
Abbreviations: XP: XerodermaPigmentosum; BCC: Basal Cell Carcinoma; SCC: Squamous Cell Carcinoma; ASCC: Acantholytic squamous cell carcinoma; EMA: Epithelial Membrane Antigen; CECT: Contrast Enhanced Computed Tomogram
Introduction
Acantholytic squamous cell carcinoma (ASCC) first described by Lever in 1947 is a rare variant of squamous cell carcinoma (SCC) characterized by features of acantholysis of cell nests resulting in a pseudoglandular appearance [1,2]. (synonyms: adenoid SCC, angiosarcoma-like SCC, pseudovascular adenoid SCC, pseudoangiosarcomatouscarcinoma, adenoacanthoma) [3]. It accounts for 3-4% of all squamous cell carcinoma types and most commonly seen in the sun exposed areas of skin particularly of elderly with a male preponderance [1,2]. Here we report a case of young female with XerodermaPigmentosum presenting as a destructive lesion of the midface which on further evaluation was found to be Acantholytic squamous cell carcinoma.
Case Report
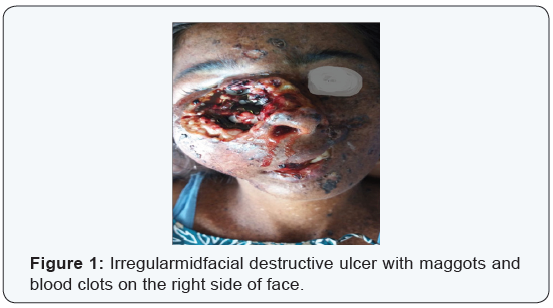
A 26 year old female presented to the ENT outpatient department with 8 days history of bleeding from the nose. She also had complains of continuous dull aching pain from a slow growing ulcer present on the right side of her face. It was associated with foul smelling discharge. The ulcer has been present since one year which started as a small swelling, later ulcerated and progressed to the present size (Figure 1). Clinical examination revealed an ulcerative lesion about 5x6 cms, irregular in shape with everted edges. Floor of the ulcer showed pale granulation tissue with maggots and blood clots. Foul smelling serosanguinous discharge was present. Surrounding skin showed hyperpigmented exfoliative lesions. Patient also had skin lesions all over her body which was present since her childhood.
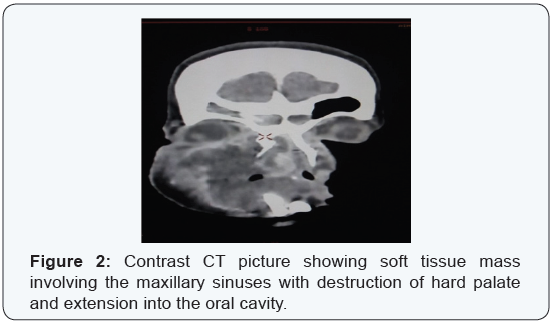
On diagnostic nasal endoscopy a friable mass was seen filling the right nasal cavity extending to left side with septal involvement. It was sensitive to touch and bled on touch. Examination of the oral cavity revealed a 2x3 cms swelling present over the hard palate, tender, firm and with well defined margins. Multiple biopsies were taken from edges of ulcer, nasal component of the mass and palatal lesion and were sent for histopathological examination. There were no palpable neck nodes Patient was examined by a dermatologist and was diagnosed to have xerodermapigmentosum. Family history of similar skin lesions was present in her cousins. Biopsy from the suspicious lesions from patient’s forehead and back were also taken. All routine hematological and biochemical investigations were normal including HIV, HBsAg and VDRL. Chest film and USG abdomen were normal [4,5]. A contrast enhanced computed tomogram (CECT) showed an ill defined soft tissue density lesion involving the right maxillary sinus extending into the nasal cavity causing destruction of walls of maxillary sinuses, perforation of hard palate with extension into the oral cavity and also extending to the right orbit (Figure 2).
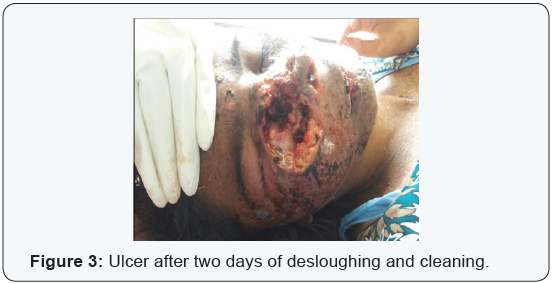
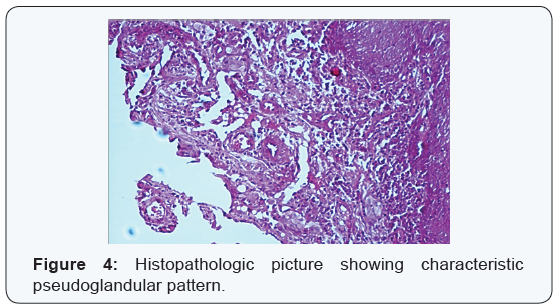
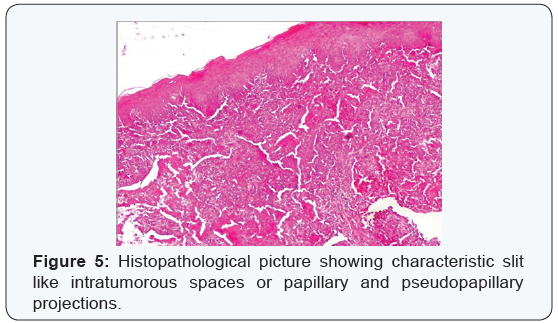
Patient was then started on intravenous broad spectrum antibiotics as well as non steroidalanti inflammatory drugs. Regular debridement of infected ulcer of the midface with removal of maggots was done. On day 4 wound was free of maggots and granulating floor was seen (Figure 3). Patient had marked improvement of pain and facial swelling. There were no further episodes of bleeding from nose. Histopathological examination of biopsy from ulcer edges, nasal mass and palatal swelling revealed final hisopathological diagnosis of rare variant of squamous cell carcinoma known as acantholytic squamous cell carcinoma (Figures 4 & 5). Histopathological examination of second biopsy specimen taken from other skin lesions over forehead and back showed features of basal cell carcinoma. Patient was advised radical surgery and reconstruction for her midface lesion. Patient refused for any curative treatment and was referred for palliative treatment.
Discussion
For any progressive destructive lesion of the midface involving the nose, paranasal sinuses and oral cavity the first provisional diagnosis that comes to our mind is Lethal Midline Granuloma. However in this case the patient had features of XerodermaPigmentosum (XP) because all the three characteristic features of XP namely freckles, photosensitivity and photophobia were present .This existing skin condition widens our provisional diagnosis since these skin lesions can predispose to a variety of skin cancers including basal cell carcinoma(BCC), squamous cell carcinoma (SCC) and even Malignant melanoma. Our first differential diagnosis was BCC as it happens to be the most common skin cancer in patients with XP. Other supporting factors were the site of the ulcer as well as the history of progression, which started as a nodule and later got ulcerated and developed to the present condition. Second condition that had to be ruled out was SCC, the next common malignant condition of the skin [6]. The everted edge and history also favored a squamous variant. Occasionally we even come across patients whose mid facial lesions cannot be classified in any particular group despite all investigations .Such patients are described as suffering from an entity called “idiopathic midline destructive disease”.
Acantholytic squamous cell carcinoma is a tumor which was initially considered to arise from the sweat glands [2] due to its tubular and gland like structures was later found to be a distinct variant of SCC. There are very few cases of ASCC documented so far and no specific risk factors have yet been identified. Some proposed predisposing factors include previous scars, burns, human papilloma virus infections, UV radiation and even immuno suppression. The earlier thought of acantholytic actinic keratosis as a precursor lesion is abandoned now since no exact association was found [5]. A documented case of ASCC originating in patients with chronic lymphocytic leukemia is also present. It is most often found on the head and neck region of elderly, but other sites of origin also have been reported including the vulva, penis, nasopharynx, and breast [7-13]. Intraoral ASCC should be differentiated from angiosarcoma due to its histopathologic similarity with the later.
Conclusion
While epitheliodangiosarcoma shows positivity for endothelial markers, ASCC stains positive for only epithelial membrane antigen (EMA) and cytokeratins [7]. Overall the mortality of ASCC compared to SCC is higher, the high mortality rates mainly due to refusal of treatment or a reporting bias. The suggested treatment protocol is wide excision of the lesion with a safe margin with prophylactic neck dissection, followed by adjuvant radiotherapy with chemotherapy wherever needed.
References
- Le Boit PE, Weedon D, Sarasain A (2006) (Eds.), Pathology and genetics of skin tumours. World Health Organization classification of tumours. IARC Press, Lyon, Europe.
- Lever WF (1947) Adenoacanthoma of sweat glands, Carcinoma of sweat glands with glandular and epidermal elements: report of four cases. Arch Derm Syphilol 56(2): 157-171.
- Barnes L, Reichart P, Sidransky P (2005) (Eds.), Pathology and genetics of head and neck tumors. World Health Organization classification of tumours. IARC Press, Lyon, Europe.
- Kusafuka K, Ebihara M , Ishiki H, Takizawa Y, Iida Y, et al. (2006) Primary adenoid squamous cell carcinoma of the oral cavity. Pathol Int 56(2):78-83.
- Johnson WC, Helwig EB (1966) Adenoid squamous cell carcinoma (adenoacanthoma). A clinicopathologic study of 155 patients. Cancer 19(11): 1639-1650.
- JCassarino DS, Derienzo DP, Barr RJ (2006) Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. Part one. J Cutan Pathol 33(3): 191-206.
- Underwood JW, Adcock LL, Okagaki T (1978) Adenosquamous carcinoma of skin appendages (adenoid squamous cell carcinoma, pseudoglandular squamous cell carcinoma, adenocanthoma of sweat gland of Lever) of the vulva: a clinical and ultrastructural study. Cancer 42(4): 1851-1858.
- Lasser A, Cornog JL, Morris JM (1974) Adenoid squamous cell carcinoma of the vulva. Cancer 33(1): 224-227.
- Watanabe K, Mukawa A, Miyazaki K, Tsukahara K (1983) Adenoid squamous cell carcinoma of the penis.Report of a surgical case clinically manifested with rapid lung metastasis. Acta Pathol Jpn 33(6): 1243-1250.
- Takagi M, Sakota Y, Takayama S, Ishikawa G (1977) Adenoid squamous cell carcinoma of the oral mucosa: report of two autopsy cases. Cancer 40(5): 2250-2225.
- Zaaatari GS, Santoianni RA (1986) Adenoid squamous cell carcinoma of the nasopharynx and neck region. Arch Pathol Lab Med 110(6): 542- 546.
- Eusebi V, Lamovec J, Cattani MG, Fedeli F, Millis RR (1986) Acantholytic variant of squamous cell carcinoma of the breast. Am J Surg Pathol 10(12): 855-861..
- Zaatari GS, Santoianni RA (1986) Adenoid squamous cell carcinoma of the nasopharynx and neck region. Arch Pathol Lab Med 110(6): 542- 546.





























