Current State of Oil and Gas Refining: Air Pollution, and Mitigation Technologies
Khalid S Al-Zahrani*
Department of Environmental Protection, Senior Environmental Engineer, Saudi Aramco, Saudi Arabia
Submission: August 09, 2025;Published:November 20, 2025
*Corresponding author:Khalid S Al-Zahrani, Department of Environmental Protection, Senior Environmental Engineer, Saudi Aramco, Saudi Arabia
How to cite this article:Khalid S Al-Zahrani. Current State of Oil and Gas Refining: Air Pollution, and Mitigation Technologies. Eng Technol Open Acc 2025; 6(4): 555695.DOI: 10.19080/ETOAJ.2025.06.555695
Abstract
The oil and natural gas industry is a leading modern industry nowadays, providing a wide range of products that are essential in social construct. Despite the critical importance of the oil and gas industry in providing some of the most needed everyday essentials that human beings depend on today, it is crucial to recognize the effort exerted in terms of fund allocation, research and development, technology implementation, and process enhancement to protect the environment and human health by using various methodologies to monitor, quantify, and mitigate air emissions. For example, the use of infrared cameras and drones in operating facilities to detect and alert in case of any leakages. All of these efforts aim to minimize the emissions associated with the life-cycle processes of the oil and gas industry in adhering to, and often championing, international standards and regulations. As a result of the efforts of the oil and gas industry, the air emissions (including GHGs and criteria pollutants like SOx and NOx) generated during the production and refining processes, including flaring, can be captured and treated in a sustainable way for disposal or converted into more valuable products, thereby achieving high returns. This paper discusses air emissions from the oil and gas industry and demonstrates how the industry mitigates the effects of such emissions. It specifically focuses on the emissions and mitigation measures of sulfur oxides (SOx), nitrogen oxides (NOx), and greenhouse gases (GHGs), along with the detection and mitigation measures of gas leakages from different equipment.
Keywords:Oil and Gas; Air Pollution Control; Mitigation Technology
Introduction
Petroleum products have been used for centuries as fuels and lubricants, but their usage surged dramatically during the late 18th and early 19th centuries due to increasing industrial demand [1]. The oil and gas sector played a pivotal role in transforming modern civilization from the Industrial Revolution to the present, enabling the rapid development of downstream industries such as transportation, lighting, and manufacturing [2]. The sector operates through the systematic extraction, processing, transportation, and utilization of carbon-rich compounds [3]. The generation and mitigation of key air pollutants, specifically sulfur oxides (SOₓ), nitrogen oxides (NOₓ), and greenhouse gases (GHGs), which mainly include methane (CH₄) and carbon dioxide (CO₂). It also highlights the technologies and regulatory frameworks employed to monitor and control these emissions. When sulfur-rich fuels are burned, sulfur is released into the atmosphere in the form of sulfur oxides. Historically, these emissions have caused serious environmental events such as the London smog in 1952 and the Donora, Pennsylvania disaster in 1948. At elevated concentrations, SOₓ can damage vegetation and aquatic ecosystems through acid rain formation and contribute to reduced air visibility due to the generation of particulate matter [4]. At high concentrations, SOx can damage the plants and wildlife through acid rain and cause the formation of particulate matter that reduces visibility [5]. Recent mitigation efforts include reducing the sulfur content in fuels through desulfurization processes. For example, the U.S. Environmental Protection Agency (EPA) established stringent standards to limit sulfur content in diesel fuel to 15 parts per million (ppm) and regulate sulfur levels in fuels used in refineries [6]. Sulfur is primarily removed via hydrodesulfurization (HDS) in catalytic reactors, and hydrogen sulfide (H₂S) is extracted from lighter gases using absorption methods. By the year 2000, desulfurization was implemented in approximately 40% of global fuel production and continues to expand. The elemental sulfur recovered in this process is typically captured using the Claus process [7].
Nitrogen oxides (NOₓ) represent another significant category of air pollutants and include various oxidized nitrogen species formed primarily through high-temperature combustion. NOₓ emissions are generated from both point and non-point sources. Approximately 50% originates from mobile sources such as vehicles, 20% from electricity generation, and 30% from industrial processes including cement production, nitric acid manufacturing, steel plants, and oil refineries [8]. NOₓ is produced when hydrocarbons combust in the presence of oxygen, and it forms via two main mechanisms: fuel NOₓ, which originates from nitrogen within the fuel itself, and thermal NOₓ, which forms from the oxidation of atmospheric nitrogen (N₂) at elevated combustion temperatures [9]. Once in the atmosphere, NOₓ can react with volatile organic compounds (VOCs) to form ground-level ozone (O₃) and fine particulate matter (PM2.5), both of which are hazardous to human health and the environment.
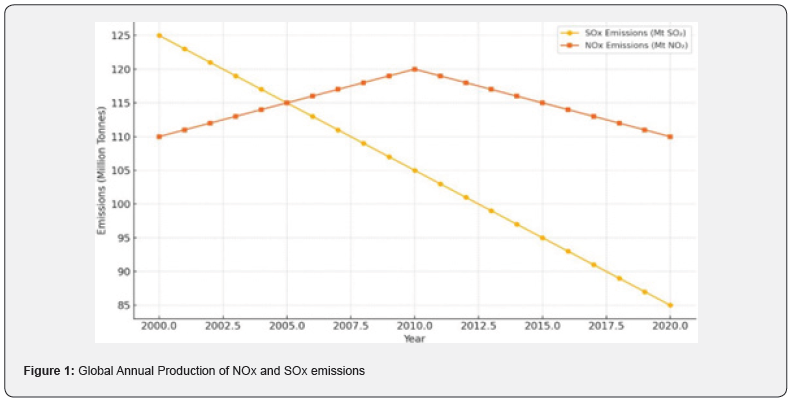
Greenhouse gases (GHGs) constitute a broad group of heattrapping gases in the atmosphere. In addition to NOₓ, which has already been discussed, the most common GHGs include carbon dioxide (CO₂), methane (CH₄), and fluorinated gases such as hydrofluorocarbons, perfluorocarbons, sulfur hexafluoride, and nitrogen trifluoride. These gases originate from similar combustion processes, but also from agriculture, land use changes, and residential and commercial activities [10]. From (Figure 1), the trend of both SOx and NOx global emission are depicted. From 2000 to 2020, global emissions of SOx showed a steady decline, primarily due to the legal implementation of desulfurization technologies, stricter international fuel standards (such as the EPA’s ultra-low sulfur diesel mandate and IMO’s 0.5% sulfur cap), and a shift from coal to lower carbon fuels like natural gas and renewables. In contrast, NOx emissions initially rose slightly and plateaued around 2010–2015 before gradually declining, reflecting the more complex nature of NOx control in high-temperature combustion systems. Subsequent reductions were driven by the adoption of advanced technologies, such as selective catalytic reduction (SCR) and the enforcement of tighter automotive and industrial emission regulations [11,12]. The objective of this paper is to systematically analyze the generation, control, and management of air emissions within the oil and gas industry, with a particular focus SOₓ, NOₓ, and GHGs. This study aims to (i) elucidate the primary sources and mechanisms responsible for the formation of these key pollutants along the oil and gas value chain; (ii) evaluate the effectiveness of current emission control technologies and regulatory frameworks; and (iii) assess the latest innovations and best practices for monitoring and mitigating both process and fugitive emissions. Through this comprehensive examination, the paper seeks to provide both a critical understanding of the sector’s environmental impact and actionable insights for further reducing its atmospheric emissions.
Processes & Emissions
Industrial production processes lead to the emission of SOx, NOx, and GHGs. The final chemical form of these pollutants is influenced by several factors, including the extent of incomplete combustion, the sulfur content of the fuel, and the specific chemical reactions occurring during synthesis [13-15]. To properly manage emissions released into the atmosphere, any control device must account for these parameters to maximize the capture of SOx, NOx, and GHGs, while ensuring compliance with environmental and public health regulations. There are established procedures for monitoring and reporting pollutant concentrations to ensure that, in the event of an issue, emissions can be adjusted accordingly [16]. The combustion of sulfur-containing fuels results in the release of SOx from both stationary and mobile sources. Due to their relatively high concentrations compared to other sulfur oxides, SOx emissions are often referred to specifically as sulfur dioxide (SO₂) or sulfur trioxide (SO₃). However, sulfur trioxide (SO₃) typically accounts for less than 1% of the total SOx emissions produced from coal combustion [17-19]. Sulfur trioxide (SO₃) can also form through the further oxidation of sulfur dioxide (SO₂) in the atmosphere.
This secondary reaction leads to the formation of sulfuric acid (H₂SO₄), a major contributor to acid rain, which negatively impacts soil quality, freshwater ecosystems, and infrastructure by accelerating corrosion. Additionally, sulfate aerosols (SO²⁻), a byproduct of atmospheric SO₂ oxidation, are key precursors to fine PM2.5 [20]. These particles can reduce atmospheric visibility and are harmful when inhaled, penetrating deep into the lungs and contributing to respiratory and cardiovascular diseases. From a life-cycle environmental analysis perspective, SO₂ and its oxidation products (SO₃ and SO₄²⁻) are classified as high-impact pollutants due to their acidification potential, toxicity to aquatic and terrestrial life, and human health risks. NOx production from oil and gas refining comes from two main sources: power generation and thermal operations required for refining. NOx pollutants can take five categorical forms, nitrogen oxide (NO) or dinitrogen dioxide (N2O2), nitrous oxide (N2O), nitrogen trioxide (N2O3), nitrogen dioxide (NO2) or dinitrogen tetroxide (N2O4), and dinitrogen pentoxide oxide (N2O5). The form of nitrogen can vary significantly as it approaches its final state while it undergoes a series of redox reactions during the processing stage and then in the atmosphere. Like NOx, the most important sources of CH4 and CO2 among other greenhouse gases in refineries are combustion process boilers, heaters, thermal oxidizers and burners [21,22]. The volatile emissions from equipment leaks represent significant emission sources in addition to those produced by combustion processes, and this GHG emissions is typically categorized into three scopes: direct emissions from refinery operations (Scope 1), indirect emissions associated with purchased electricity generation (Scope 2), and indirect emissions resulting from the use of downstream products sold (Scope 3). This classification framework helps ensure comprehensive accounting and management of emissions throughout the entire value chain [23].
Control Technologies
The oil and gas industry are increasingly adopting the best available technologies (BAT) and employing various techniques to monitor and quantify emissions to comply with international standards and environmental regulations. Mitigation measures are implemented to prevent or reduce air emissions at their source. To specifically address SO₂ emissions, the U.S. Environmental Protection Agency (EPA) introduced key regulatory limits: in 2006, the sulfur content of diesel fuel was capped at 15 parts per million (ppm), and by 2013, the sulfur content of fuels used in boiler applications was restricted to a maximum of 0.5% by weight. These standards aim to significantly reduce SOx emissions and their harmful impacts on human health and the environment [24,25].
(Table 1) shows a series of control technologies reported in literature. One of the most common techniques is absorption. Temperature Swing Adsorption (TSA), Pressure Swing Adsorption (PSA), and sorbent injection are the adsorption techniques that offer advantages in cost, deployment ease and high maturity [26]. TSA and PSA use solid adsorbents to capture SOx, with TSA relying on heat and PSA on pressure changes to regenerate the material. These systems are compact, water-free, and suitable for low- to mid-concentration SOx streams, making them attractive for decentralized or modular installations [27].
Fuel desulfurization is a critical step in reducing SOx emissions before combustion, primarily achieved through hydrodesulfurization (HDS), where sulfur compounds in fuels are removed by reacting with H2 over a catalyst (i.e., Fe, Pd, Pt). In the post-combustion (downstream processes), two main flue gas desulfurization (FGD) methods are widely used, namely wet and dry [28,29]. Wet FGD involves scrubbing flue gases with a slurry (e.g., MgO or CaO) offering high SO₂ removal efficiency (>90%). However, it requires significant water usage which is not environmentally benign. In contrast, dry FGD methods, including semi-dry and dry sorbent injection (DSI), use powdered sorbents without generating wastewater, making them more suitable for smaller or water-limited installations. On the whole, dry systems are less efficient than wet systems, since they offer lower capital and operational costs and simpler operation [30].
Oxy-fuel combustion is an emerging technique, particularly suited for hard-to-abate sectors [31]. In this process, fuel is burned in a mixture of pure O2 and recycled flue gas instead of air, resulting in a flue gas with CO₂ and H2O vapor. This significantly simplifies CO₂ capture, as the gas stream is already highly concentrated with higher partial pressure, reducing the need for complex separation steps. Oxy-fuel combustion offers several advantages, such as higher thermal efficiency and ease of integration with post CO2 capture. While technology has shown strong potential in cement and steel industries, its application in oil and gas is still under development due to challenges like high oxygen production costs and retrofitting requirements. NOx emissions in refinery operations occur through Selective catalytic reduction (SCR) or Selective Non-catalytic reduction (SNCR), processes through which NOx are reduced to N2 and H2O [32,33]. SCR is the more advanced and widely used of the two flue gas treatment methods, which involves the addition of ammonia (NH3) in the presence of a titanium oxide- vanadium pentoxide catalyst. This process can achieve a destructive removal efficiency (DRE) of up to 94%. Selective non-catalytic reduction (SNCR) uses ammonia or urea compounds without a catalyst, which saves on the cost of these expensive components but requires a higher operating temperature.
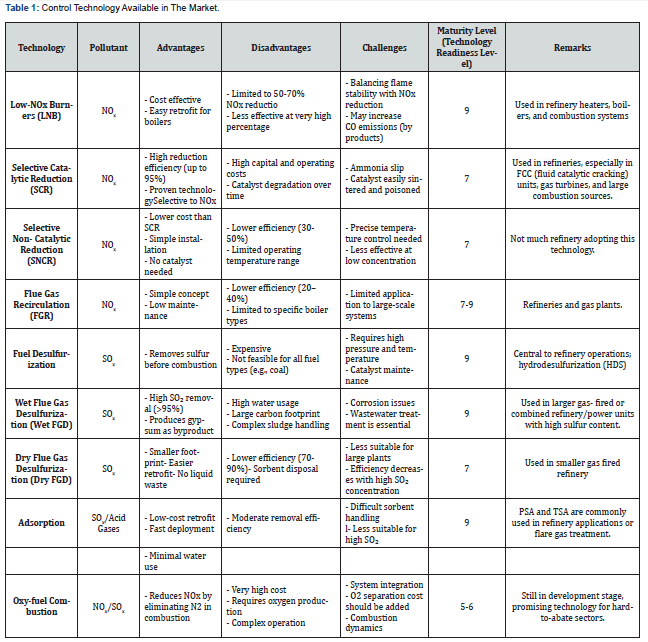
Low-NOx Burners (LNB) and Flue Gas Recirculation (FGR) are two effective combustion control strategies used in industrial and power generation settings to reduce NOₓ emissions [34]. LNBs achieve NOₓ reduction (typically 30–50%) by staging the combustion process, lowering the flame temperatures and O2 concentration, respectively, making them a cost-effective and easy-to-retrofit solution [35]. In contrast, FGR recirculates a portion of the flue gas back into the burner to dilute the O2 concentration, offering higher NOₓ reduction potential (up to 70%), but requiring more complex infrastructure. New paradigm, integration of both into one system can further enhance the NOx reduction in combustion systems. LNBs reduce NOx formation by staging fuel injection and lowering flame temperatures, while FGR works by redirecting a portion of cooled flue gas back into the combustion chamber, diluting O2 levels and further reducing peak flame temperatures. When integrated, these technologies complement each other, leading to a positive synergistic effect that can achieve NOx reductions of over 70% [36]. This combined method is particularly useful in industrial boilers and furnaces, offering a practical and efficient approach to meeting stringent emission standards without complex infrastructure.
Overall, the deployment of these control technologies in industrial environments is driven not just by performance, but by a balance of operational practicality, cost, efficiency, and regulatory environments. Wet flue gas desulfurization (FGD), while recognized for its high SO₂ removal efficiency and reliability in large-scale operations, carries significant drawbacks—namely, intensive water and energy demand, complex waste management, and high capital outlay. This restricts its suitability to centralized facilities able to absorb these costs and administrative burdens. Conversely, dry and semi-dry FGD methods provide a more flexible, lower-cost solution suitable for distributed or modular installations and regions facing water scarcity. Yet, their lower SO₂ removal efficiency can be a limiting factor where regulations are rigorous or exhaust streams are concentrated, and frequent sorbent handling introduces long-term sustainability questions.
Hydrodesulfurization (HDS) remains crucial for upstream emission reduction, especially in refinery settings, but its reliance on hydrogen supply and precious metal catalysts adds operational complexity and cost. Deep desulfurization, increasingly mandated by fuel standards, can raise these costs further, and the technology offers no solution for post-combustion sources or distributed emissions. meanwhile, Oxy-fuel combustion stands out for its capacity to simplify CO₂ capture and elevate overall thermal efficiency, making it particularly promising in hard-to-abate sectors like steel and cement. Nonetheless, its adoption in oil and gas has lagged, in part due to high oxygen production expenses and the challenging retrofitting of existing infrastructure-a tradeoff that preserves its role mainly for future-proof or pilotlevel projects rather than widespread industrial application.
For NOx control, selective catalytic reduction (SCR) sets the benchmark for removal efficiency, reliably achieving industryleading NOx reductions in refineries and power stations. Yet, it depends on costly catalysts and requires stringent operational controls to avoid secondary emissions (e.g., ammonia slip), meaning that maintenance demands and safety protocols can be intensive. Selective non-catalytic reduction (SNCR), while less capital-intensive and simpler to implement, typically delivers only moderate NOx abatement, with effectiveness highly contingent on temperature regimes found in specific combustion systems. Low- NOx Burners (LNB) and Flue Gas Recirculation (FGR) provide upstream, process-integrated solutions that exploit combustion staging and flue gas cooling to minimize NOx formation before treatment is needed. These approaches are simple to retrofit and cost-effective, making them attractive for existing industrial sites seeking compliance upgrades. However, improper configuration can decrease combustion efficiency or promote other undesired emissions. When combined, LNB and FGR yield a synergistic effect, allowing NOx reductions surpassing 70% without major infrastructure overhaul, a compelling pathway for industrial boiler and furnace operations under tightening standards.
Ultimately, the optimal selection of emission control technology is driven by a nuanced assessment of site-specific requirements, regulatory pressure, resource availability, and operational risks. As the oil and gas sector transitions to lower emissions, strategic integration of these technologies—tailored to the technical and regulatory context of each facility—remains critical for balancing environmental goals with economic and practical realities
Regulations
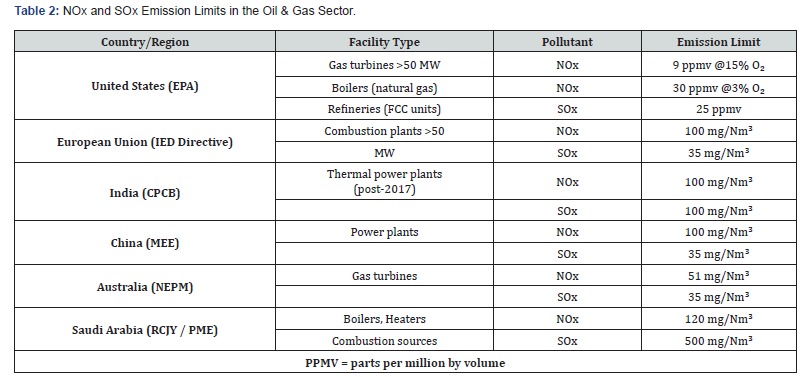
The World Health Organization (WHO) and U.S. Environmental Protection Agency have published global maximum recommendation values for several important air pollutants, which are related to oil and gas industry (Table 2) [37-41]. There are SO2 emission standards for refining operations such as sulfur recovery units
(SRUs), furnaces and boilers, and cells using the Claus process or other regeneration systems. To control these emissions, regulatory bodies impose emission limits. For instance, 35 mg/ Nm³ for natural gas-fired units in the EU or up to 500 mg/Nm³ in Saudi Arabia when low-sulfur fuels are used. These limits drive the implementation of technologies such as fuel desulfurization, flue gas desulfurization (FGD), and tail gas treatment units (TGTUs), which are critical for compliance. Thereafter, SRUs are often equipped with incinerators or scrubbers to ensure SOx levels meet environmental standards, helping in reduce the impact of SOx on air quality, acid deposition, and public health. Meanwhile, NOx, primarily formed during high-temperature combustion in furnaces, gas turbines, boilers, and sulfur recovery units, are key pollutants in oil and gas operations due to their role in smog formation, acid rain, and respiratory health impacts. Emission limits for Nox such as 100 mg/Nm³ for gas-fired turbines under the EU Industrial Emissions Directive or 30 ppmv in U.S. refinery boilers are applied to ensure regulatory compliance and environmental protection. These limits inform operational upgrades like low-NOx burners, selective catalytic reduction (SCR), and fuel switching to reduce emissions. Their application is critical in guiding facilities toward best practices and cleaner technologies. For example, if a refinery exceeds a limit, it must retrofit abatement systems to meet permitted values and avoid penalties.
Conclusions
Oil and gas facilities involve multiple processes that emit varying levels of air pollutants, particularly nitrogen oxides (NOx) and sulfur oxides (SOx), depending on the emission source and type of fuel used. With advances in scientific understanding and pollution control technologies, the industry has made significant progress in mitigating these emissions through the implementation of Best Available Technologies detailed in Table 1 below, such as selective catalytic reduction (SCR) for NOx and desulfurization units for SOx. These measures are further supported by strict adherence to international environmental standards and regulations.
Moreover, the integration of real-time monitoring tools such as infrared cameras, drones, and remote sensing systems has proven effective in detecting and managing emissions, enabling rapid intervention and reinforcing environmental compliance. For instance, leak detection and repair (LDAR) sniffers can be adopted to inspect each valve and flange to detect leaks. They work to detect and locate leaks using tracer gas such as hydrogen. These tests are relatively inexpensive, but they require time and depend on user experience. Infrared cameras, known as optical gas imaging (OGI), can determine the source and concentration of a leak from up to 10 meters away. With increased computer modeling and deep learning capabilities, leaks can be better controlled to improve the effectiveness of LDAR policies. Furthermore, to reduce the leaked gases significantly from the refining processes, the drones have been used to detect and quantify the emissions from any emission sources and equipment depending on the speed and laser sensitivity. Collectively, these efforts contribute to minimizing the environmental impact of oil and gas operations while protecting ecosystem integrity and human health.
References
- Hall C, Tharakan P, Hallock J, Cleveland C, Jefferson M (2003) Hydrocarbons and the evolution of human Nature 426(6964): 318-322.
- (2018) Oil Industry, USA.
- Jafarinejad S (2016) Control and treatment of sulfur compounds specially sulfur oxides (SOx) emissions from the petroleum industry: A review. Chemistry International 2(4): 242-253.
- Hanania J (2016) Great smog of London, England.
- (2021) U.S. Environmental Protection Sulfur Dioxide Basics, USA.
- Smith S (2004) Historical Sulfur Dioxide Emissions 1850-2000: Methods and PNNL-14537.
- Sirvastava V (2012) An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC advances 2, 759-783.
- S. Environmental Protection Agency (1999) Nitrogen Oxides (NOx), Why and How They are Controlled. USA.
- S. Environmental Protection Agency (1998) Nitrogen Oxides: Pollution Prevention and Control. USA.
- S. Environmental Protection Agency (2007) Overview of Greenhouse Gases. USA.
- Xie S, Tan W, Li Y, Ma L (2022) Copper Single Atom-Triggered Niobia-Ceria Catalyst for Efficient Low-Temperature Reduction of Nitrogen Oxides. ACS Catalysis 12(4): 2441- 2453.
- Wang Y, Sun M, Zhou J, Xiong Y, Zhang Q, et , (2023) Atomic coordination environment engineering of bimetallic alloy nanostructures for efficient ammonia electrosynthesis from nitrate. Proc Natl Acad Sci U S A 120(32): e2306461120
- Syrek-Gerstenkorn S, Syrek-Gerstenkorn B, Paul S (2024) Comparative Study of SOx, NOx, 5 and PM10 in the UK and Poland from 1970 to 2020. Appl Sci 14(8): 3292.
- Anenberg S, Miller J, Minjares R, Du L, Henze D K, Forrest Lacey, et , (2017). Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 545(7655): 467-471.
- Johnston FH, Borchers-Arriagada N, Morgan GG, Jalaludin B, Palmer AJ, et , (2020). Unprecedented health costs of smoke-related PM2.5 from the 2019-20 Australian megafires. Nat Sustain 4, 42 – 47.
- Fleig D, Andersson K, Normann F, Johnsson F (2011) SO3 Formation under Oxyfuel Combustion Industrial & Engineering Chemistry Research 50: 8505-8514.
- Environmental Integrity Project (2020) Greenhouse Gases from Oil, Gas, and Petrochemical Production 1-34.
- Skowron A, Lee DS, De León RR, Lim LL, Owen B (2021) Greater fuel efficiency is potentially preferable to reducing NOx emissions for aviation’s climate impacts. nature communications 12: 564.
- Lamprou E, Stergioudi F, Skordaris G, Michailidis N, Nessi E, et al., (2024) Effect of NOX and SOX Contaminants on Corrosion Behaviors of 304L and 316L Stainless Steels in Monoethanolamine Aqueous Amine Solutions. Coatings 14(7): 842.
- Tsai JH, Lee MY, Chiang HL, et al. (2021) Effectiveness of SOx, NOx, and Primary Particulate Matter Control Strategies in the Improvement of Ambient PM Concentration in Taiwan. Atmosphere 12(4): 460.
- Liu Z, Woo SI (2006) Recent Advances in Catalytic DeNOX Science and Technology. Catalysis Reviews 48(1): 43-89.
- Niccolucci V, Marchi M, Minardi I, Nadia Marchettini (2025) Nitrogen Footprint accounting and food sustainability: Insights from the Italian wine industry. Environmental Impact Assessment Review 112(1136674): 107830.
- S. Environmental Protection Agency (2002) EPA Air Pollution Cost Control Manual. Sixth Edition, USA.
- Leverett J, Lie WH, Ali Khan MH, Ma Z, et al., (2025) Navigating the challenges of global NOx emissions throughout the energy transition: state of play and outlook. Sustainable Energy Fuels (14).
- Tartakovsky D, Kordova-Biezuner L, Broday DM, et al., (2005) PM5 and NOX concentrations decrease as a result of a railway electrification. Environmental Monitoring and Assessment 197(2): 188.
- Zhu Z, Xu, B (2022). Purification Technologies for NOx Removal from Flue Gas: A Review. Separations 9(10): 307.
- Rezaei F, Rownaghi AA, Monjezi S, Lively RP, Jones CW (2015) SOx/NOx Removal from Flue Gas Streams by Solid Adsorbents: A Review of Current Challenges and Future Directions. Energy Fuels 29: 5467-5486.
- Karimi R, Golmohammadi B (2024) Simulation and optimization of heavy fuel oil (HFO) refining platform to low sulfur marine fuel (LS-FO) by oxidative desulfurization 14(1): 29830.
- Armandsefat F, Hamzehzadeh S, Azizi N (2024) Efficient and promising oxidative desulfurization of fuel using Fenton like deep eutectic solvent (14): 12614.
- Kurniawan TA, Khan S, Mohyuddin A et al. (2024) Technological solutions for air pollution control to mitigate climate change: an approach to facilitate global transition toward blue sky and net-zero emission. Chemical Papers 78: 6843-6871.
- Wang X, Shan S, Wang Z, Zhijun Zhou & Kefa Cen et al. (2024) Review on thermal-science fundamental research of pressurized oxy-fuel combustion technology. Front Energy 18: 760-784.
- Han L, Cai S, Gao M, Hasegawa J, Wang P, et al., (2019) Selective Catalytic Reduction of NOX with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem Rev 119(19): 10916-10976.
- Cheng X, Bi XT (2014) A Review of Recent Advances in Selective Catalytic NOx Reduction Reactor Technologies. Particuology 16: 1-18.
- Kondor IP (2025) An Experimental Study of NOx Reduction with Superheated Steam Injection in Evaporative Burner Using Heating Oil. In: Baranyi P, Palkovics L, Zöldy M (eds) Proceedings of the 2nd Cognitive Mobility COGMOB 23 2023. Lecture Notes in Networks and Systems 1345 Springer Cham.
- Kopyev EP, Mukhina MA, Sadkin IS et al. (2025) Investigation of the Steam Injection Method Used to Reduce Emissions During Liquefied Petroleum Gas Combustion in an Atmospheric Burner. Arab J Sci Eng 50: 5265-5277.
- Zhong Q, Jiang Wz, Gao W, et al. (2025) Pre-reduction sintering process with flue gas recirculation for reduction alkalis harm and flue gas emission J Cent South Univ 32: 106–
- Jyethi DS (2016) Air Quality: Global and Regional Emissions of Particulate Matter, SOX, and In Plant Responses to Air Pollution, Kulshrestha U, Saxena P, Eds; Springer 5-19.
- S. Environmental Protection Agency (2025) New Source Performance Standards (NSPS) for Stationary Gas Turbines.
- (2025) European Bureau for Research on Industrial Transformation and
- (2021) WHO Air Quality Guidelines






























