Manufacturing Engineering Challenges of Pharmaceutical 3D Printing for on-Demand Drug Delivery
Ozkan T1, Ienina M2*, Barakh Ali SF3, Rahman Z3 and Mansoor Khan3
1Department of Mechanical Engineering, Texas A&M University, USA
2EOS North America, USA
3Department of Pharmacy, Texas A&M University, USA
Submission: April 25, 2018; Published: May 31, 2018
*Corresponding author: Tanil Ozkan, Department of Mechanical Engineering, Texas A&M University, College Station, TX 77843, USA, Email: tozkan@tamu.edu
How to cite this article: Ozkan T, Ienina M, Barakh Ali SF, Rahman Z, Mansoor K. Manufacturing Engineering Challenges of Pharmaceutical 3D Printing for on-Demand Drug Delivery. Eng Technol Open Acc. 2018; 1(5): 555575. 10.19080/ETOAJ.2018.01.555575
Abstract
There are about two dozen 3D printing technologies available in the market. These technologies differ in terms of layering mechanism, type of material that can be handled, binding mechanism and suitability for solid dosage form manufacturing. Based on literature information and preliminary data, fused deposition modeling (FDM), selective laser sintering (SLS) and powder-bed 3D printing methods have emerged as the most promising candidates for delivering on-demand drugs according to established Good Manufacturing Practice (GMP) guidelines of the pharmaceutical industry. However, information on interplay of critical process parameters (CPPs) and critical materials attributes (CMAs) on the critical quality attributes (CQAs) of the formulations and products that will determine their in-vitro and in-vivo performance is not readily available in the literature. Through this mini-review, we underline the pressing need for the systematic exploration of these critical factors and present the Ishikawa diagrams for the three mainstream 3D printing techniques already penetrating the specialized niches of the pharmaceutical manufacturing market at an extremely fast pace.
Keywords: Additive Manufacturing; Critical quality attributes; Compatibility; Fused filament fabrication; Micro particles; Nan particles; Ingredients
Abbrevations: AM: Additive Manufacturing; FDM: Fused Deposition Modeling; FFF: Fused Filament Fabrication; SLS: Selective Laser Sintering; PVA: Polyvinyl Alcohol; CPPs: Critical Process Parameters; CQAs: Critical Quality Attributes; CMAs: Critical Material Attributes; GMPs: Good Manufacturing Practices
Introduction
Additive Manufacturing (AM) in general and three-dimensional printing (3DP) in particular is emerging technologies expected to revolutionize pharmaceuticals manufacturing along with other fields [1-4]. These technologies offer the ability to create limitless dosage forms that are likely to challenge conventional drug fabrication methods not only in product quality and efficacy but also in cost efficiency as 3D printers have already been successful in producing novel dosage forms within minutes [3]. Three situations where this on-demand pharmacy capability may be applicable include printing directly on the implants or tissue scaffolds, printing “just in-time” in healthcare facilities or in other resource-constrained settings and printing low-stability drugs for immediate consumption. In all these scenarios, 3D printing technologies provide attractive solutions to explore on-demand pharmacy [5]. FDA approved first commercial drug product based on 3DP technology in 2015 (NDA #207958, Spritam® (levetiracetam) triturates Aprecia Pharmaceuticals Co) [6]. It is a high drug loaded tablet that disintegrates in less than 10 seconds which is atypical even for orally disintegrating tablet. Even though its underlying manufacturing technology is two decades old, very little information is available in the public domain about the process and formulation variables that could affect critical quality attributes of drug products manufactured by 3DP. Furthermore, quality defects in the drug products manufactured by 3DP will be entirely different from compressed tablets. Thus it is essential to evaluate 3DP techniques for their compatibility with pharmaceuticals from the perspective of on-demand pharmacy feasibility and application.
3DP Technologies Amenable to Pharmaceutical Manufacturing and Their Unique Aspects and Materials
The primary 3DP technologies that can be used for pharmaceuticals manufacturing are inkjet-based or inkjet powder-based 3DP, fused deposition modeling (FDM) also called fused filament fabrication (FFF) and selective laser sintering (SLS).
Inkjet-based or inkjet powder-based 3DP
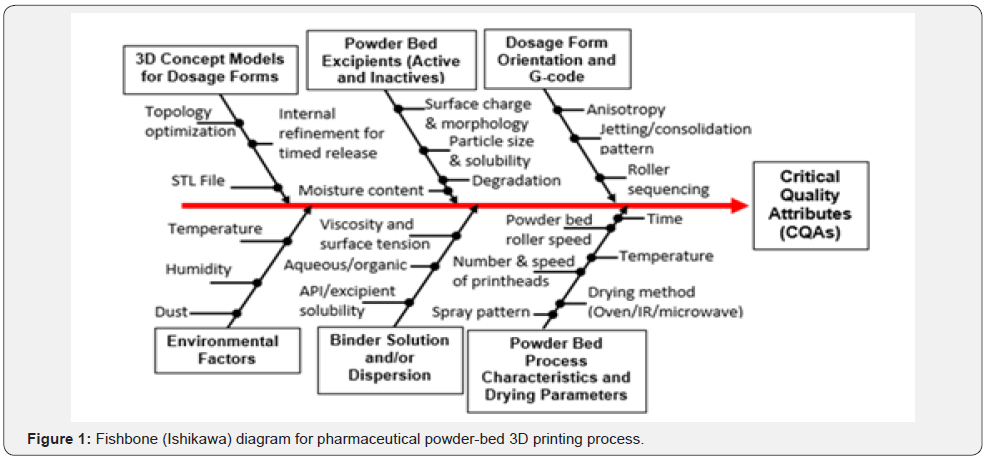
Whether another material or a powder is used as the substrate is what differentiates 3D inkjet printing from powderbased 3D inkjet printing. In inkjet-based drug fabrication, inkjet printers are used to spray formulations of medications and binders in small droplets at precise speeds, motions, and sizes onto a substrate. The most commonly used substrates include different types of cellulose, coated or uncoated paper, microporous bioceramics, glass scaffolds, metal alloys, and potato starch films, among others [2-5]. Researchers have further improved this technology by spraying uniform “ink” droplets onto a liquid film that encapsulates it, forming microparticles and nanoparticles. Such matrices can be used to deliver small hydrophobic molecules and growth factors [7]. In powder-based 3D printing drug fabrication, the inkjet printer head sprays the “ink” onto the powder foundation. When the ink contacts the powder, it hardens and creates a solid dosage form, layer by layer. The ink can include active ingredients as well as binders and other inactive ingredients. After the 3D-printed dosage form is dry, the solid object is removed from the surrounding loose powder substrate [3-5,7]. Very limited work has been reported concerning controllable printing parameters in binder jetting process and materials attributes (active and inactive), which could substantially affect critical quality attributes (CQAs) of pharmaceutical formulations. Therefore, having a good understanding and experimental insight into the practical effects of such parameters on CQAs seems to be essential. The fishbone (Ishikawa) diagram for pharmaceutical powder-bed and inkjet 3D printing processes is given in Figure 1.
Fused deposition modeling (FDM)
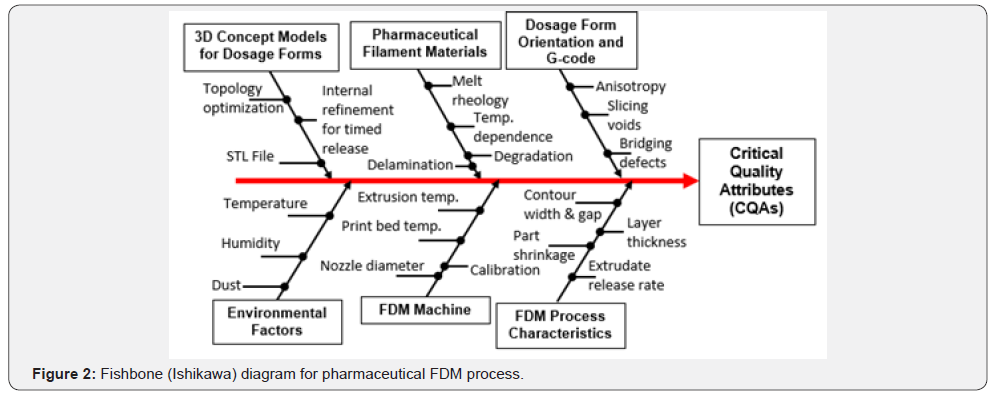
In FDM/FFF, the object is formed by layers of melted or softened thermoplastic filament extruded from the printer’s head at specific directions as dictated by computer software. Inside the FDM printer’s head the filament is heated to just above its softening point which is then extruded through a nozzle, deposited layer by layer immediately followed by solidification [4-5,7-9]. The speed of the extruder head may also be controlled to stop and start deposition and form an interrupted plane without stringing or dribbling between sections although nanoparticle emissions were detected during the process [2-3,9-12]. The potential of FDM 3D printing to incorporate drugs into commercially available filaments has been explored previously [13,14]. Nonetheless, all those studies highlighted several challenges involved in employing printing technique for pharmaceutical applications. The use of elevated temperatures (185-220 °C) and limited drug loading (0.063-9.5%w/w) renders it less suitable for many drugs particularly thermolabile ones [13-15]. FDM 3D printing has been also restricted to a number of biodegradable thermoplastic polymers such as polylactic acid (PLA) [16] and polyvinyl alcohol (PVA) [4,13- 15] in comparison to a wide variety of choices for conventional tableting. Despite the attractive properties of PVA [17] and PLA [18], the use of high polymer ratio in combination with the need for high molecular weight of the polymers generates polymeric matrices with limited porosity, thus resulting in extended drug release patterns. To increase the flexibility and potential of FDM 3D printing process, never filament formulations also employing nanocomposite compounding approaches will be needed to overcome existing limitations of FDM type pharmaceutical 3D printing. The fishbone (Ishikawa) diagram for pharmaceutical FDM processes is provided in Figure 2.
Selective laser sintering (SLS)
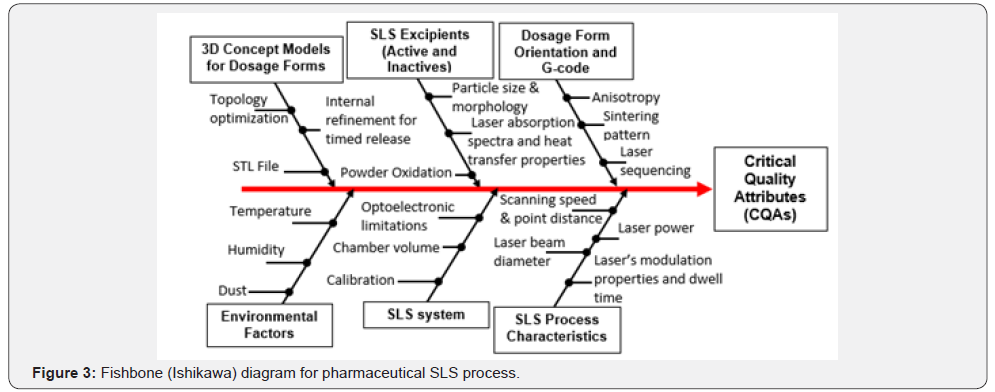
Like all methods of 3D printing, an object printed with the SLS process starts as a CAD file. Objects printed through this method are made with powder materials, most commonly plastics, such as nylon, which are dispersed in a thin layer on top of the build platform inside an SLS machine. The laser heats the powder either to just below its melting point (sintering) or above its melting point (melt consolidation), which fuses the particles in the powder together into a solid form. Once the initial layer is formed, the platform of the SLS machine drops usually by less than 0.1 mm, thus exposing a new layer of powder for the laser to trace and fuse together. This process continues again and again until the entire object has been printed [2,19]. Very limited information is available in the literature for pharmaceutical application of the SLS technique [5,20]. The characteristic limitations of this process are attributed to incompatibilities between laser energy settings and properties of thermoplastic polymer used to print drug product. It is important to understand the interplay of the SLS critical process parameters (CPPs) and polymeric powder critical material attributes (CMAs) on critical CQAs, as shown by the Ishikawa diagram in Figure 3.
Conclusion
The most promising 3DP technologies for pharmaceutical applications and their manufacturing engineering related challenges were discussed. In all methods, understanding critical process parameters (CPPs) is equally important as understanding critical material attributes (CMAs) to ensure consistent quality of 3D printed solid dosage form. Rigorous experimental studies are needed to validate process and materials engineering correlations between CMAs and CPPs, which can also identify optimal control strategies and potential risk factors for process failure or deficiencies in terms of pharmaceutical good manufacturing practices (GMPs). Including all variants and hybrid approaches, about two dozen 3DP technologies currently exist in the market each with varying capabilities and complexities. Although objective assessments of current process limitations and capabilities assign a higher chance to powder bed and SLS techniques in the race for on-demand drug delivery, the Darwinian dynamics of technology evolution coupled with process engineering and materials technology innovations will determine which particular ones would be indeed the fittest to survive in the domain of pharmaceutical manufacturing.
References
- Schubert C, van Langeveld MC, Donoso LA (2014) Innovations in 3D printing: a 3D overview from optics to organs. Br J Ophthalmol 98(2):159-161.
- Gibson I, Rosen D, Stucker B (2015) Additive manufacturing technologies, 3D printing, rapid prototyping, and direct digital manufacturing (2nd edn). Springe, USA.
- Kalaskar DM (2017) 3D Printing in Medicine. Woodhead Publishing; Duxford, UK.
- Chia HN, Wu G, Weiss TL, Gu BK, Wardyn JD, et al. (2016) 3D Printing in Medicine. Scientific Research Publishing, China
- Ventola CL (2014) Medical applications for 3D printing: Current and projected uses. Pharm Ther 39(10):704-711.
- Spritam (2018) (levetiracetam) tablets label.
- Ursan I, Chiu L, Pierce A (2013) Three-dimensional drug printing: a structured review. J Am Pharm Assoc 53(2): 136-144.
- Hoy MB (2013) 3D printing: making things at the library. Med Ref Serv Q 32(1):94-99.
- Azimi P, Zhao D, Pouzet C, Crain NE, Stephens B (2016) Emissions of ultrafine particles and volatile organic compounds from commercially available desktop three-dimensional printers with multiple filaments. Environ. Sci. Technol 50(3):1260-1268.
- Jin Y, Plott J, Shih AJ (2015) Extrusion-based additive manufacturing of the moisture cured silicone elastomer, International Solid Freeform Fabrication Symposium, Laboratory for Freeform Fabrication and the University of Texas, Austin, TX, USA, pp.308-318.
- Kim Y, Yoon C, Ham S, Park J, Kim S, et al. (2015) Emissions of nanoparticles and gaseous material from 3D printer operation. Environ. Sci. Technol 49(20): 12044-12053.
- Steinle P (2016) Characterization of emissions from a desktop 3D printer and indoor air measurements in office settings. J. Occup. Environ. Hyg 13(2): 121-132.
- Goyanes A, Buanz AB, Hatton GB, Gaisford S, Basit AW (2015) 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm 89:157-162.
- Skowyra J, Pietrzak K, Alhnan MA (2015) Fabrication of extendedrelease patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci 20(68): 11-17.
- Goyanes A, Buanz ABM, Basit AW, Gaisford S (2014) Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm 476(1-2): 88-92.
- Sandler N, Salmela I, Fallarero A, Rosling A, Khajeheian M, et al. (2014) Towards fabrication of 3D printed medical devices to prevent biofilm formation. International journal of pharmaceutics 459(1-2): 62-64.
- Morita R, Honda R, Takahashi Y (2000) Development of oral controlled release preparations, a PVA swelling controlled release system (SCRS): I. Design of SCRS and its release controlling factor. J Control Release 63(3): 297-304.
- Hamad K, Kaseem M, Deri F, Ko Young G (2016) Mechanical properties and compatibility of polylactic acid/polystyrene polymer blend. Mater. Lett 164: 409-412.
- Shirazi SFS, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HSC et al. (2015) A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci Technol Adv Mater 16(3): 033502.
- Norman J, Madurawe RD, Moore CM, Khan MA, Khairuzzaman A (2017) A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev 108: 39-50.






























