“Wait And See” Approach for Rectal Adenocarcinoma
Daniele Candini, Margarita Martín Martín and Fernando López Campos
Department of Radiation Oncology, Ramón y Cajal University Hospital, Spain
Submission: February 12, 2017; Published: February 27, 2017
*Corresponding author: Fernando López Campos, Radiation Oncology Department, Ramón y Cajal University Hospital, Ctra. Colmenar Viejo Km. 9,100, 28034, Madrid, Spain, Tel: +34 663158959; Email: flcampos@salud.madrid.org
How to cite this article: Daniele C, Margarita M M, Fernando L C.“Wait And See” Approach for Rectal Adenocarcinoma. Canc Therapy & Oncol Int J.2017; 3(3): 555613. DOI: 10.19080/CTOIJ.2017.03.555613
Abstract
Neoadjuvant chemoradiotherapy followed by surgery has been the standard of care to treat patients diagnosed with locally advanced rectal cancer. Some patient achieves a complete pathological response to chemo radiotherapy and the outcomes in this setting are especially promising. The role of surgery in patients with pathologic complete response is being questioned due to significant morbidity and long-term effects on quality of life. That is why there is an interest in a "watch and wait” approach in patients who achieve a clinical complete response with neoadjuvant treatment with the goal of omitting surgery and allowing for organ preservation. Despite this, a clinical complete response does not always mean a pathological complete response, so better clinical or imaging resources are needed to identify which patients can safely undergo a "wait and see” approach. This mini-review resumes the current data on non-operative management and controversies associated with this approach.
Keywords: Wait and see; Rectal cancer; Pathological complete response; Organ preservation; Quality of life
Abbreviations: CCR: Colorectal Cancer; LARC: Locally Advanced Rectal Cancer; TME: Total Mesorectum Excision; pCR: Complete pathological Response; cCR: Complete clinical Response; QoL: Quality of Life; 5-FU: 5-Fluoro-Uracil
Introduction
Colorectal cancer (CCR) is the most frequently occurring tumor in our country with more than 32,000 new cases per year, and about 40% of these are located in the rectum, being the second cause of cancer death in both genders. Neoadjuvant treatment with long-acting radio-chemotherapy in patients diagnosed with locally advanced rectal cancer (LARC) followed by surgery with total mesorectum excision (TME) after 8 weeks (± 2 weeks) has demonstrated its superiority over other therapeutic modalities in terms of efficacy (significant reduction of local-regional recurrences) and toxicity [1].
This benefit is balanced by an increased risk of postoperative complications, including a postoperative death rate of 28%, which may reach a postoperative morbidity rate of 30% at 6 months in elderly patients (> 85 years), in addition to an important longterm impact on anorectal, urinary and sexual function [2]. In relation to comorbidity, surgical intervention has a negative impact on the quality of life of these patients [2], considering that patients with low rectal tumors require a permanent ostomy depending on the surgical technique used, with the consequences that it involves.
Recently there was a growing awareness of the delicate balance between healing and quality of life, and this is why the role of radical surgery for all patients with rectal cancer is increasingly questioned. In addition, a complete pathological response (pCR), defined as the total absence of viable residual tumor cells in the surgery specimen, is sometimes observed after preoperative radio-chemotherapy. In a review of phase II and III studies, a total ypCR ratio of 13.5% was identified, although this value has been increased in later series with the use of higher doses of radiotherapy (> 54 Gy) [3] and / or with the optimization of the chemotherapy treatments [4]. At this time, physicians are trying to identify predictive factors of ypCR after the administration of standard neoadjuvant treatment, in order to enhance these responses, to arise a new conservative attitude from a surgical point of view called "wait and see” approach: in other words, to not proceed to immediate surgery in such patients after the end of the radio-chemotherapy.
The largest clinical review that supports such a therapeutic approach for patients who achieve a complete clinical response (cCR), defined as the absence of clinically detectable residual tumor, comes from a Brazilian series [5-10] of retrospective studies, including patients recruited from 1991 to 2013; the results of these studies suggest that observation with periodic controls in certain groups of patients leads to similar overall survival rates to that of patients who underwent radical surgery with subsequent confirmation of a pCR, eliminating morbidity and mortality of abdominoperineal resection . Further studies of other groups support this data [11,12].
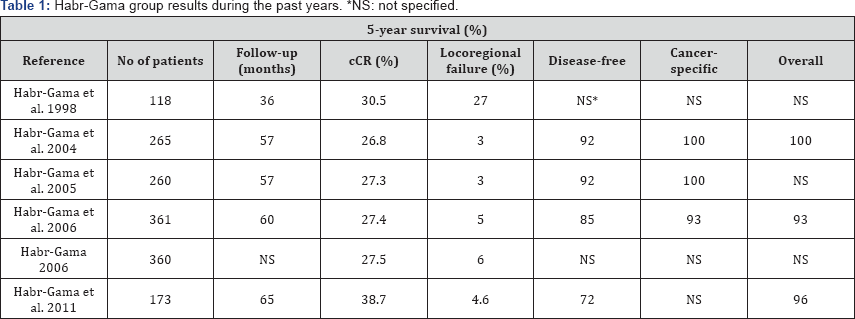
The following Table 1 shows the results of the experience of Habr-Gama et al., with a local recurrence rate of 4.6%, disease-free survival at 5 years of 72%, and overall survival at 5 years of 96% [13]. Nowadays, with the data which has been published, the proportion of patients with different neoadjuvant radio-chemotherapy regimens reaches a cCR range between 10.9% and 38.7% [14], determining an important percentage of patients that could benefit from this approach after selecting the better candidates depending on a multiple set of variables.
Discussion
Patients Selection
Patients selected for this type of approach were diagnosed of LARCs, located in the lower third of the rectum (up to a maximum of 7 cm from the anal margin), without limitations in tumor size (from T1 to T4, although the rate of 58%, 28%, 16% and 12% of cT1, cT2, cT3 and cT4, respectively, appears to indicate that the success of the wait and see approach is conditioned by the size of the initial tumor). Lymph node involvement was considered a limiting factor for entry into this type of observation regimen, considering that only those patients staged as N1 according to the AJCC classification were included [15].
cCR Definition
Historically, the definition of cCR has been inconsistent. Most of these proposals defined cCR as the absence of detectable tumor in a clinical examination or rectal examination and endoscopy [16]. Recently, Habr-Gama et al. [17] redefined and standardized the definition of cCR. This is characterized by: 1) a clear absence of palpable tumor by rectal examination, and 2) the endoscopic absence of residual tumor, or only a small ulceration or eschar with negative biopsy. Regarding the second point, the presence of a deep ulcer, palpable nodules or a significant stenosis are NOT criteria of cCR; while YES they are a slightly whitish mucosa, the presence of telangiectasias or loss of flexibility of the mucosal wall as a consequence of the neoadjuvant treatment. From the radiological point of view, cCR is characterized by the absence of a tumor in the pelvic MRI (low signal in the diffusion sequences or presence of various stages of fibrosis), in addition with absence of local edema in the abdomino-pelvic CT. The role of PET/CT in the re-staging of rectal cancer after receiving neoadjuvant treatment is unclear, although some studies estimates a global diagnostic accuracy of this technique around 96% [18].
cCR Timing
Published papers [11,12,19] have not clearly defined the appropriate time interval for the definition of a cCR, and the data range from a minimum of 6 weeks to a maximum of 14 months. Up to three determinants have been identified that influence an increase in the number of pCRs: the increasing range between neoadjuvant radiotherapy and surgery, escalation of radiotherapy doses above 50-54 Gy, and neoadjuvant additional chemotherapy (which by itself increases the time to surgery) [19]. Given these factors, the definition of possible cCR should not be made before 8 weeks after the end of the radio-chemotherapy treatment [19].
Follow-Up
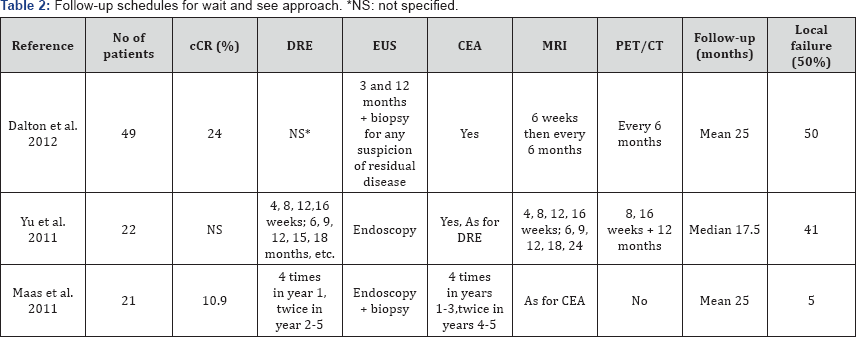
Only 6 studies have published the follow-up timetable. Habr- Gama et al. [10] in their last published series, established an intense follow-up protocol, including a monthly clinical visit with digital rectal examination (DRE), rectoscopy (EUS), CEA levels and CT during the first three months, with subsequent spacing of controls until one year of treatment with radio-chemotherapy (at which time a cCR is considered into account) [10]. Appelt et al. [20], followed up with a clinical and endoscopic examination every 2 months during the first year, every 3 months in the second year, every 6 months in the third year, and every 12 months during the fourth and fifth year. In addition, a PET/CT scan was performed 3 times during the first year, 2 times during the second year and successively once a year. Other examples of follow-up can be found in the following table (Table 2) [11-13,21].
Quality of Life
The improvement in the quality of life (QoL) of patients who did not undergo surgery is evident, compared to the group of patients who presented pCR after surgery, with a lower Wexner score of incontinence (0.8 vs 3.5) and defecation frequency (1.8 vs 2.8 times/day) [12]. In a recent study by Renehan et al. [22] (OnCoRe project), the percentage of colostomy free survival at 3 years was first evaluated in a group of patients with cCR + wait and see approach vs another group of patients undergoing surgical resection for failure to achieve a cCR with a statistically significant result in favour of the wait and see group (74% vs 47%). In the study by Appelt et al. [20]the impact of treatment on QoL was measured through the EORTC QoL questionnaire (QLQ- CR29), which was completed by each patient before and after radio-chemotherapy, at 6 and 12 months of follow-up and then annually, showing small variations in long-term follow-up.
Radiotherapy
The series of Habr-Gama et al. [21] initially used the dose of 50.4 Gy in 28 fractions. More recently, the dose was increased to 54 Gy in 32 fractions (18). Other studies used 45-50 Gy [21]. The techniques and field size used have not been described in most cases, but it is assumed that the upper edge of the radiotherapy field is at the L5-S1 level. The Danish study by Appelt et al. [3] has achieved the highest cCR rate described so far (78%) with a 1-year local recurrence rate of 15%. This result is explained by the fact that the dose of radiotherapy used was much higher than that of the other series: an initial dose of 60 Gy was used with external radiotherapy followed by an endorectal brachytherapy boost of 6 Gy. These data suggest that the dose of radiotherapy administered may be a factor to be taken into account to achieve an increase of the cCR, also considering the toxicity associated with it.
On the other hand, only a small randomized study compared the percentage of ypCR obtained with a short course of radiotherapy (5 x 5 Gy) with deferred surgery vs conventional radiotherapy (50.4 Gy with 5-fluorouracile), being statistically superior the group of conventional radio-chemotherapy (13% vs 3%) in the evaluation of pCR [23,24]. This result suggests that the 5 x 5 Gy (short course) is not optimal if the target is an increase of the cCR. The RAPIDO Phase III study (NCT01558921), currently under recruitment, randomizes patients with LARCs with high risk factors to receive a short course of radiotherapy treatment followed by chemotherapy (CAPOX x 6 cycles or FOLFOX4 x 9 cycles) vs conventional radio-chemotherapy (50.4 Gy with capecitabine): the results of this trial will provide additional data on the role of neoadjuvant intensifying chemotherapy along with short course radiotherapy in potentially increase the number of pCR.
Chemotherapy
The chemotherapy regimens used are variable, based mainly on the use of concomitantly administered 5-Fluorouracil (5-FU) or fluoropyrimidines, with an improvement in the percentage of ypCR and locoregional control [25,26] in these patients. Some recent studies suggest new strategies in this regard, but nowadays none of them can be considered as a realistic option for LARCs because most of these regimens described an increase in adverse effects that is not accompanied by a clinically relevant improvement in cCR or pCR rates. Therefore, fluoropyrimidine- based chemotherapy with 5-FU or, alternatively, oral capecitabine should still be considered as the standard scheme most commonly used in routine clinical practice for neoadjuvant treatment of rectal cancer [27]. We do not have data regarding the use of adjuvant chemotherapy in patients with cCR, regardless of tumor size or nodal involvement. Only in a series published in 2013 by Habr-Gama et al. (54 Gy with 3 cycles of 5-FU and leucovorin) three more chemotherapy cycles identical to the previous ones were added, showing a cCR rate at 1 year of 57% and a local failure rate at 1 year of 17% [28].
Salvage Therapy
The local recurrence rate in patients with initial cCR with the wait and see approach can be as high as 31%, if early failures (<12 months) are grouped with late failures. In these cases, surgical salvage treatment is a safe option that can be performed in 90% of recurrences, leading to a good local control (94%) of the disease, with 78% of organ preservation [29]. The study by Renehan et al. [22] confirms and supports this data, adding that up to 90% of local recurrences are in the rectal lumen, an ideal location for salvage resections.
Future Perspectives
Several clinical trials on this treatment strategy are currently under investigation. The study (NCT01047969), coordinated by "The Royal Marsden Hospital”, is now into recruitment with two primary objectives: 1) to estimate the percentage of patients who can avoid surgery, defined as the percentage of patients who were not operated and those who had a cCR 2 years after radiochemotherapy; 2) to demonstrate the safety of deferred surgery, defined as the percentage of patients with local failure at 2 years (positive margin in the resected tumor or disease that can't be rescued).
The multicenter clinical trial "NCT02438839” will investigate whether a boost with external radiotherapy up to 66 Gy can replace brachytherapy boost operated by Appelt et al. [3], trying to maintain the high cCR rates achieved by them and with a lower rate of side effects. On the other hand, in October 2015 the recruitment of patients for the European Network of "wait and see" began in Denmark; new centers will be added during the current year. Finally, we are awaiting the results at 3 and 5 years of the prospective observational study of the Danish Colorectal Cancer Group (NCT00952926) where was examined the 1-year local failure rate of radiochemotherapy in patients with nonoperated low rectal tumors [20].
Conclusion
The omission of surgery in patients with cCR has obvious short-term advantages, such as lower morbidity, colostomies, sequelae, and postoperative complications. Nevertheless, many of the studies cited have limitations, since they mainly are small retrospective studies with short and heterogeneous follow-up schemes. The results of QoL and functional improvement have been reported only in two of the publications. Undoubtedly, the findings of the latest published studies indicate that the wait and see strategy for patients with rectal cancer located in the lower third who achieve a cCR with radio chemotherapy can be a safe and effective alternative in selected cases.
However, many unresolved doubts remain, such as: 1) how to establishing the intensity and the duration of clinical, radiological and possibly pathological follows-up; 2) the requirement or not of adjuvant chemotherapy depending on the initial clinical stage; 3) the strategies for the fiable diagnosis of the largest number of cCR as well as the follow-up of the same ones. Wait and see approach is, therefore, a therapeutic strategy that should be considered within the usual clinical practice in selected cases, due to the lack of multicenter prospective studies where the results could be reproduced and extrapolated in order to extend its use.
References
- sauer R, Becker H, Hohenberger W, R Ödel C, Wittekind C, et al. (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17): 1731-1740.
- Paun BC, Cassie S, MacLean, Dixon E, Buie WD (2010) Postoperative complications following surgery for rectal cancer. Ann Surg 251(5): 807-818.
- Appelt AL, PlØen J, Harling H, Frank S Jensen, Lars H Jensen, et al. (2015) High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 16(8): 919-927.
- Hartley A, Ho K, McConkey C, Geh JI (2005) Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br J Radiol 78(934): 934-938.
- Habr-Gama A, de Souza PM, Ribeiro U, Nadalin W, Gansl R, et al. (1998) Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum 41(9): 1087-1096.
- Habr-Gama A, de Souza PM, Ribeiro U (2001) Multimodality therapy in low rectal cancer: long-term outcome of complete responders. Dis Colon Rectum 44: A18.
- Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, et al. (2004) Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long term results. Ann Surg 240(4): 711-717.
- Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, et al. (2005) Long term results of preoperative chemoradiation for distal rectal cancer: correlation between final stage and survival. J Gastrointest Surg 9(1): 90-109.
- Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, et al. (2006) Patterns of failure and survival for non-operative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg 10(10): 1319-1328.
- Habr-Gama A (2006) Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Colorectal Dis 8(Suppl 3): 21-24.
- Dalton R, Velineni R, Osborne M, Thomas R, Harries S, et al. (2012) A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis 14(5): 567-571.
- Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, et al. (2011) Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 29(35): 4633-4640.
- Glynne-Jones R, Hughes R (2012) Critical appraisal of the 'wait and see' approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg 99(7): 897-909.
- Habr-Gama A, Perez RO, Sao Juliao GP, Proscurshim I, Gama-Rodrigues J (2011) Nonoperative approaches to rectal cancer: a critical evaluation. Semin Radiat Oncol 21(3): 234-249.
- Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, et al. (2010) Longterm outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11(9): 835-844.
- Wynn GR, Bhasin N, Macklin CP, George ML (2010) Complete clinical response to neoadjuvant chemoradiotherapy in patients with rectal cancer: opinions of British and Irish specialists. Colorectal Dis 12(4): 327-333.
- Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, et al. (2010) Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum 53(12): 1692-1698.
- Habr-Gama A, Gama-Rodrigues J, Perez RO, Proscurshim I, Sao Juliao GP, et al. (2008) Late assessment of local control by PET in patients with distal rectal cancer managed nonoperatively after complete tumor regression following neoadjuvant chemoradiation. Tech Coloproctol 12(1): 74-76.
- Habr-Gama A, Perez RO, Sabbaga J, Nadalin W, Sao Juliao GP, et al. (2009) Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum 52(12): 1927-1934.
- Appelt AL, PlØen J, Vogelius IR, Bentzen SM, Jakobsen A (2013) Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys 85(1): 74-80.
- Yu SK, Brown G, Heald RJ (2011) Deferral of rectal surgery following a continued response to preoperative chemoradiotherapy (Watch andWait) study: a phase II multicenter study in the United Kingdom. J Clin Oncol 29(Suppl 4): abstract 489.
- Renehan AG, Malcomson L, Emsley R (2016) Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 17(2): 174-83.
- Latkauskas T, Pauzas H, Gineikiene I, Janciauskiene R, Juozaityte E, et al. (2012) Initial results of a randomized controlled trial comparing clinical and pathological downstaging ofrectal cancer after preoperative shortcourse radiotherapy or long-term chemoradiotherapy, both with delayed surgery. Colorectal Dis 14(3): 294-98.
- Nilsson PJ, van Etten B, Hospers GA, Pâhlman L, van de Velde CJ, et al. (2013) Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancerthe RAPIDO trial. BMC Cancer 13: 279.
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, et al. (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355(11): 1114-1123.
- Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, et al. (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 24: 4620-4625.
- Marijnen CA (2015) Organ preservation in rectal cancer: have all questions been answered? Lancet Oncol 16(1): e13-e22.
- Habr-Gama A, Sabbaga J, Gama-Rodrigues J, Sao Juliao GP, Proscurshim I, et al. (2013) Watch and Wait Approach Following Extended Neoadjuvant Chemoradiation for Distal Rectal Cancer: Are We Getting Closer to Anal Cancer Management? Dis Colon Rectum 56(10): 11091117.
- Habr-Gama A, Gama-Rodrigues J, Sao Juliao GP, Proscurshim I, Sabbagh C, et al. (2014) Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 88(4): 822-28.






























