ISO Biocompatibility Evaluations of Glutaraldehyde Cross-linked Amniotic Membranes
Kimberly Velarde1, Connor Stewart1, Sarah Berglund1, Audrey Arvonen1 and Robert B. Diller1,2*
1Amnio Technology, LLC, Phoenix, Arizona
2Northern Arizona University, Department of Biology, Flagstaff, AZ, USA
Submission: May 13, 2024; Published: May 24, 2024
*Corresponding author: Robert B. Diller, Amnio Technology Phoenix, Arizona and Northern University, Department of Biology, Flagstaff, AZ, USA.
How to cite this article: Kimberly Velarde, Connor Stewart, Sarah Berglund, Audrey Arvonen and Robert B. Diller*. ISO Biocompatibility Evaluations of Glutaraldehyde Cross-linked Amniotic Membranes. Curr Trends Biomedical Eng & Biosci. 2024; 22(4): 556098. DOI:10.19080/CTBEB.2024.22.556098
Abstract
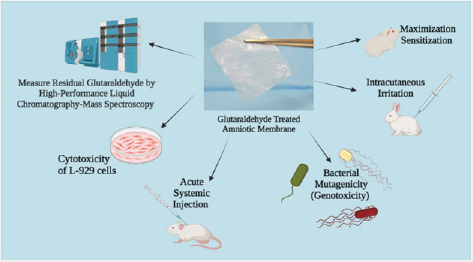
Amniotic membrane, the innermost layer of the placenta, is biocompatible through standards defined by the International Organization for Standardization (ISO) when used as a biological medical device. A battery of tests per ISO-10993:2012 was completed using test article extracts to assess the biocompatibility of glutaraldehyde cross-linked amniotic membrane. High-Performance Liquid Chromatography-Mass Spectroscopy (HPLC-MS) was done to measure toxic concentrations of glutaraldehyde on the amniotic membrane, resulting in no traces of residual glutaraldehyde. An assessment of L-929 cells treated with the test article extract showed a score of “0”, “1”, and “2” at 24/48/72-hour marks. An acute systemic injection test was performed using test article extracts injected into ten albino Swiss mice, concluding no significant biological response up to 72 hours post-treatment. Moreover, a bacterial mutagenicity test (genotoxicity) against the test article extracts using five strains of bacteria detected no increase in reversion rates, and samples were considered non-mutagenic. Intracutaneous injections of the test extract were administered on three albino rabbits to evaluate skin sensitization; these showed no intradermal irritation. A maximization sensitization test was conducted by injecting test article extracts into five different injection sites of guinea pigs. After being challenged, no abnormal clinical signs were detected, and the test article was not considered an intracutaneous irritant. The results obtained in this study indicated glutaraldehyde-treated amniotic membranes are biocompatible using the ISO guidelines and are appropriate for use as biologically derived medical devices.
Keywords: Biocompatibility testing; Amniotic membranes; Glutaraldehyde; International Organization for Standardization; Toxicological effects
Abbreviations: ISO: International Organization for Standardization; HPLC: High-Performance Liquid Chromatography-Mass Spectroscopy; AM: amniotic membranes; HCT/P: human cells, tissues, or cellular tissue-based products; GA: Glutaraldehyde; ECM: extracellular matrix; TGF-β: transforming growth factor β; PHS: Public Health and Safety; 1X-PBS: 1X Phosphate-Buffered Saline; GLP: Good Laboratory Practice; DNPH: Dinitrophenylhydrazine; APCI: Atmospheric Pressure Chemical Ionization; IACUC: Institutional Animal Care and Use Committee; AAALAC: Association for Assessment and Accreditation of Laboratory Animal Care; HDPE: High-Density Polyethylene; ZDEC: Zinc Diethyldithiocarbamate; SLS: Sodium Lauryl Sulfate
Introduction
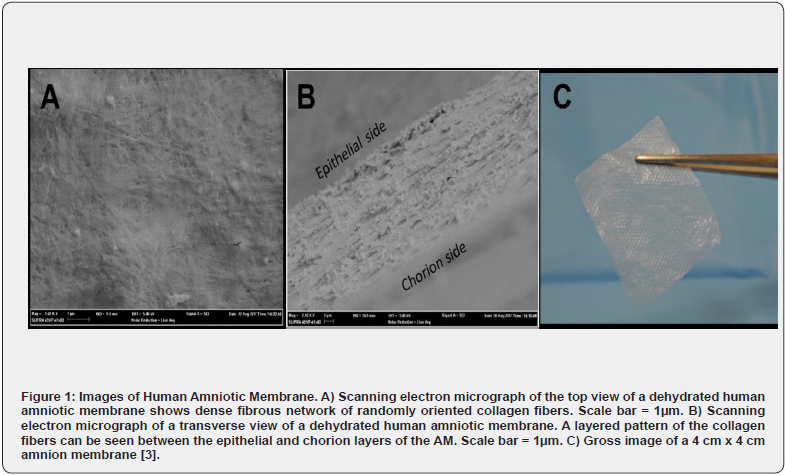
The clinical utility of human amnion-derived tissue has been recognized, particularly in wound management, for over 100 years. A survey of current clinical trials in the U.S. suggests an increasing demand for the potential applications of amniotic membranes in ulcerative wound care, ophthalmic conditions, neurologic and gynecologic conditions, and dental abnormalities [1]. In the United States, amniotic membranes (AM) are regulated as human cells, tissues, or cellular tissue-based products (HCT/P) under title 21 of the Code of Federal Regulations, part 1271 (21CFR1271) and section 361 of the Public Health and Safety (PHS) Act. Amnio Technology, LLC is a company that has been utilizing the benefits provided by these AMs for wound healing for over a decade. Ultimately, the increasing use of AM is due to being primarily composed of extracellular matrix (ECM) protein fibers and glycosaminoglycans, which promote cellular migration and proliferation [2-3]. These AM scaffolds provide a uniquely human-derived 3-D matrix which is a semipermeable barrier, providing physical support while regulating cellular activity. As hydrated, native tissue, AMs vary in thickness between 35-60 μm. Once these membranes are decellularized and dehydrated, they measure 10-30 μm thick and appear like cellophane (See Figure 1). The structural organization of AM allows it to be an ideal candidate for crosslinking as it is primarily composed of a dense fibrous network of collagen fibrils [2].
Clinically, AM has several beneficial effects described in the literature, such as: favorably influencing wound healing by inducing epithelialization, providing an anti-inflammatory and analgesic effect, and possessing both anti-scarring and antiadhesive properties due to the downregulation of transforming growth factor β (TGF-β) and TIMP activity [4-7,8-10]. Moreover, AM has an anti-microbial effect due to the expression of molecules like ß-defensins and elafin along with anti-inflammatory effects that can be partly attributed to the production of factors such as hyaluronic acid, IL-1 receptor agonist, and IL-10 [3,8,12-13]. Many studies reveal that AMs are immunotolerant, biocompatible, and have suitable mechanical properties (permeability, stability, elasticity, flexibility, and resorbability) ideal for tissue regeneration [11,14]. In addition, AM has low antigenicity and is known to modulate angiogenesis, having both pro- and anti-angiogenic properties [3-5,8,14,15]. All these assets make amniotic membranes the ideal platform for clinical use.
In tissue engineering, scaffold formation through tissue regeneration is essential to prevent patients from experiencing infection, scarring, pain, and discomfort. In wound healing, the AM helps create a scaffold that mimics the host’s epithelial ECM structure and aids in re-epithelization. For example, collagen is an abundant protein in the human body that acts as an organized framework of biomechanical and biochemical functions in multiple systems, such as the skin, blood vessels, tendons, cartilage, and bone [16]. Collagen types I, III, IV, V, VI, and XV have been identified in AM, providing stability, cell adhesion, and proliferation during wound healing [17]. In fact, to take advantage of these well-defined properties, AM can be crosslinked to slow the resorption rate while increasing its handling characteristics and durability. Many studies have been performed on AMs to determine these biological characterizations, but none have used these HCT/Ps and put them through the battery of tests required of medical devices.
Glutaraldehyde (GA) is a well-characterized protein crosslinker. In collagen-rich tissues, like AM, GA induces covalent bonds between and within collagen fibrils, increasing biomechanical strength and resistance to proteolytic enzymes [2]. Crosslinking collagen with glutaraldehyde involves reacting with the free amine groups of lysine or hydroxylysine residues of the polypeptide chains [9,18]. The increase in biomechanical strength of collagen proteins offers mechanical improvement by structurally modifying the AM. For example, force-elongation measurements of glutaraldehyde-treated AM have been reported to increase by 175% compared to untreated amniotic membranes [19]. Despite contributing to tissue engineering, GA needs to be evaluated as it is used in numerous applications varying in domains. To illustrate, GA has been used as a disinfectant for industrial antimicrobial applications like hospitals and water treatments, as a biological tissue fixative, and as x-ray film processing, moreover, it aids in decreasing dentin hypersensitivity and sterilizing surgical instruments [20]. GA is also used as a pesticide, and can be found in pain, leather tanning, laundry detergent, embalming fluid, oil, and gas recovery [21].
Besides testing positive for multiple bacterial mutagenicity tests against various Salmonella and Escherichia coli strains, GA has been shown to have a highly transdentinal cytotoxic effect when used in combination with other dental products for the treatment of dentin hypersensitivity [18,19]. Despite the multiple valuable uses of glutaraldehyde, the human body can absorb GA through respiratory, oral, and dermal routes, and safety precautions must be taken [23]. Glutaraldehyde causes mild to severe irritation to tissues it encounters, such as the nose, eyes, pharynx, and skin, although severity depends on the concentration and duration of exposure [23,24]. For this purpose, HCT/Ps cross-linked with GA must be evaluated for their potentially harmful effect on human tissues.
For this study, AM was crosslinked with GA, and to assess the toxicity, the membranes were evaluated for residual GA in addition to biocompatibility testing. Furthermore, various tests per international standards were completed to define whether amniotic membranes treated with glutaraldehyde are toxic. Since the allograft is in-tended to be applied to the wounds, the recommended endpoints for consideration per 10993-1, attachment A, Table A.1 for a surface contacting device with prolonged expo-sure (>24 hours to 30 days) are cytotoxicity, sensitization, and irritation or intracutaneous reactivity. Two additional endpoints were evaluated to determine if the chemically crosslinked allografts influenced genotoxicity and acute systemic toxicity. Implantation evaluations were not performed on animals, given these allografts have been used clinically for over a decade. The results of these evaluations will help determine the safe applications of glutaraldehyde-treated amniotic membranes as a biologically de-rived surface-contacting medical device.
This article is the first publication to provide ISO 10993 testing to a human cell or tissue product, which requires no testing per FDA guidelines, to support its use as a medical device.
Materials and Methods
Preparation of Cross-linked Amnion Membranes
Amniotic membranes were obtained after voluntary cesarean sections. Per our standard operating procedures, blunt dissection removed the chorion from the amnion. The amnion was submerged into a 0.1% glutaraldehyde solution for several minutes under agitation and then washed in sterile 1X phosphatebuffered saline (1X PBS) twice under agitation then rinsed twice with sterile normal (0.9%) saline. Membranes were allowed to completely dry at room temperature before being cut to size. AM were packaged into individual pouches and underwent terminal gamma sterilization with a dose range of 15.8 – 28 kilogray (kGy).
Good Laboratory Practice Statement
All experiments were conducted by WuXi AppTec (St. Paul, MN). Each study re-ported hereafter was conducted under Good Laboratory Practice (GLP) regulations, FDA 21 CFR part 58 Good Laboratory Practice for Nonclinical Safety Studies. Per regulations, a Quality Assurance unit provided oversight for adherence to standard operating procedures and study protocols. In addition, animal studies were conducted in compliance with International Standards as described. The extraction preparation of samples and controls for all assays were performed in compliance with ISO-10993-12:2012 Biological Evaluation of Medical Devices 12: Sample Preparation and Reference Materials.
Analysis of Residual Glutaraldehyde in an Amnion Membrane by HPLC-MS
The evaluation of residual glutaraldehyde in the membranes was performed using HPLC-MS. The test article extracts were prepared by submerging either one 8 cm x 8 cm or two 4 cm x 8 cm crosslinked AM allografts in 2.0mL of LC-MS grade water. Test articles were incubated for 24±2 hours at 37±2°C. After extraction, a sample of each test article extract was acidified, diluted with acetonitrile, and derivatized with 2,4-dinitrophenylhydrazine (DNPH) for 30 min at room temperature with gentle agitation. Appropriate controls (pure water and un-derivatized glutaraldehyde in water) were prepared similarly. HPLC-MS analyzed prepared solutions and a glutaraldehyde standard in water. Samples were compared against a calibration curve of DNPH-derivatized glutaraldehyde in acetonitrile (0.25-1.0 μg).
Analysis was performed on a Dionex UltiMate3000 UHPLC connected to a Dionex Surveyor MSQ Plus. Separation was performed on an Agilent Poroshell 120 SB-C18 (2.1 mm x 100 mm, 2.7 μm) (Agilent Technologies, USA) column at 40°C with an injection volume of 5 μL and a flow rate of 0.20 mL/min. The isocratic mobile phase consisted of 30% water and 70% acetonitrile. To ionize samples, atmospheric pressure chemical ionization (APCI) was in negative mode with a cone voltage of 20 V, a dwell time of 1 second, and a probe temperature of 400°C was used.
Ethical Statement and Animal Welfare
WuXi AppTec holds the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International accreditation. Additional guidelines followed (versions current at the time of the study): NIH guidelines, “Guide for the Care and Use of Laboratory Animals”, National Research Council of the National Academies, eighth edition, 2011; OPRR, “Public Health Service Policy on Humane Care and Use of Laboratory Animals”, Health Research Extension Act of 1985; USDA Department of Agriculture, Animal and Plant Health Inspection Service, 9 CFR, Parts 1, 2, and 3, Animal Welfare, Final Rule 1989; WuXi AppTec policy on Humane Care. Protocol number 98-02F, amendments, or procedures involving the care or use of animals were reviewed and approved by an Institutional Animal Care and Use Committee (IACUC).
Extraction Conditions and Methods
All extractions for ISO evaluations were performed per ISO 10993-12:2012, section 10.3, Table 1. The allografts were treated as films or sheets with a thickness less than 0.5 mm which required 6 cm2/mL. This was chosen over membranes with irregularly shaped porous devices (low-density materials), such as membranes and textiles requiring 0.1 g/mL. The average weight of 1 cm2 of AM allograft is 0.82 ± 0.08 mg. For this method, approximately 122,000 cm2 would be necessary to create a 0.1 g/mL extraction. This amount of membrane in 1 mL of extraction medium would not be feasible. Since the porosity of an AM is negligible, as evidenced in Figures 1A and 1B, it was determined that porosity would not influence the surface area calculations of the methods used. The extraction for the cytotoxicity evaluation was performed at 37 ± 1°C for 24 ± 2 hours per note following the listed extraction conditions for using tissue culture media.
Cytotoxicity Using L-929 Mouse Fibroblast Cells
This assay was performed in compliance with the following international standards: ISO 10993-5:2009. Cultures of L-929 cells (mouse fibroblast) (Lot: 102213) were obtained from the American Type Culture Collection (ATCC # CCL-1). Mycoplasmafree cell lines were purchased from the vendor and kept frozen per the manufacturer’s recommendations. The L-929 cells were only sub-cultured for up to 15 passages to maintain the sensitivity and then discarded. Cultures were grown and used as monolayers in disposable tissue culture labware at 37±1°C in a humidified atmosphere of 5±1% CO2 in air at WuXi AppTec.
Extraction Media, Test Article, and Control Preparation
The extraction vehicle was prepared using Eagle’s minimal essential medium (E-MEM), supplemented with 5% (v/v) fetal bovine serum (FBS), and was used to extract the test article. The medium was also supplemented with the following: 2 mM L-glutamine, 10 mM HEPES, 0.01 mg/mL vancomycin, 0.01 mg/ mL gentamicin, 1% 1000 units/mL penicillin, and 1% 2.50 ug/mL amphotericin B (Fungizone). The pH was confirmed to be 7.33.
The partial test article remained intact, was placed into an extraction vessel, and prepared at a ratio of 120 cm2 to 20 mL of extraction vehicle. The extraction mixture and controls were then incubated for 24 ± 2 hours at 37 ± 1 °C. At the start of the extraction, the solutions appeared clear and free of particulates. The extracts were agitated during and at the end of the extraction period. After extraction, the test article was intact with no macroscopically observable degradation, particulate-free, clear, and normal in color. The extract was not filtered before use and was used immediately.
A negative, positive, and cell control were run parallel to the test article. A negative control (high-density polyethylene (HDPE)) known to be non-toxic under the test conditions was prepared at a ratio of 60 cm2 to 20mL of extraction vehicle. A positive control (polyurethane film containing 0.1% zinc diethyldithiocarbamate (ZDEC)) known to be toxic under the test conditions was prepared at a ratio of 120 cm2 to 20 mL of extraction vehicle. A cell control (E-MEM + 5% FBS) was incubated in parallel with the test sample and controls.
Experimental Procedure
L-929 cells were plated at a density of 1.0 x 104 cells/cm2 into a 6-well plate at least 24 ± 2 hours before use. Prior to inoculation, the cell cultures were examined to ensure they had formed a nearly confluent monolayer, appeared uniform, and viable. The test article and controls were extracted with the appropriate volume of E-MEM + 5% FBS for 24 ± 2 hours at 37 ± 1°C. At the end of the extraction period, the vessels were well shaken, and the extraction media was used immediately. Next, 1.0 mL aliquots of the test article and control extracts were plated in triplicate onto the cell line and were incubated in a humidified atmosphere for 72 ± 4 hours. The cell monolayers were evaluated for cytotoxic effects at 24, 48, and 72 ± 4 hours. At each incubation period, the cell cultures were observed for signs of cytopathic development, including lysis, crenation, plaques, and excessive rounding of cells.
Test Evaluation
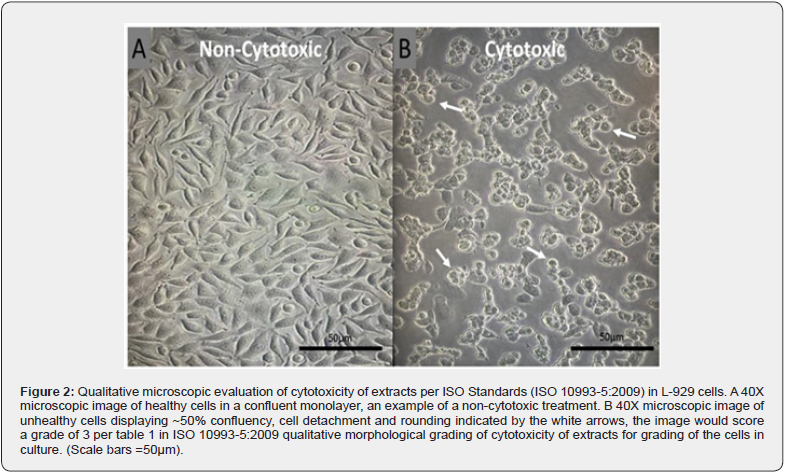
Criteria for evaluating cytotoxicity included morphologic changes in cells, such as granulation, crenation, rounding, and loss of viable cells from the monolayer by lysis or detachment. The validity of the test requires that negative control cultures maintain a healthy normal appearance throughout the test. Figure 2 shows a qualitative microscopic representation of non-cytotoxic and cytotoxic L-929 cells.
Acute Systemic Injection Test
This assay was performed in compliance with the following international standards: ISO 10993-11:2006.
For extraction, the test article was placed in sterile vessels and prepared at a ratio of 120cm2 to 20mL of extraction vehicle (0.9% Normal Saline). The extraction mixtures and corresponding control blanks were incubated for 72 ± 2 hours at 37 ± 2 °C. At the start of the extraction, the solutions appeared clear and free of particulates. The extracts were agitated during and after the extraction period, and the liquid was aseptically decanted into a sterile vessel. The test article was observed after all extractions to be intact with no macroscopically observable degradation, clear in transparency, free from particulates, and normal in color. After decanting, the unfiltered extracts were maintained at room temperature and used within 24 hours of preparation.
Test Article Administration
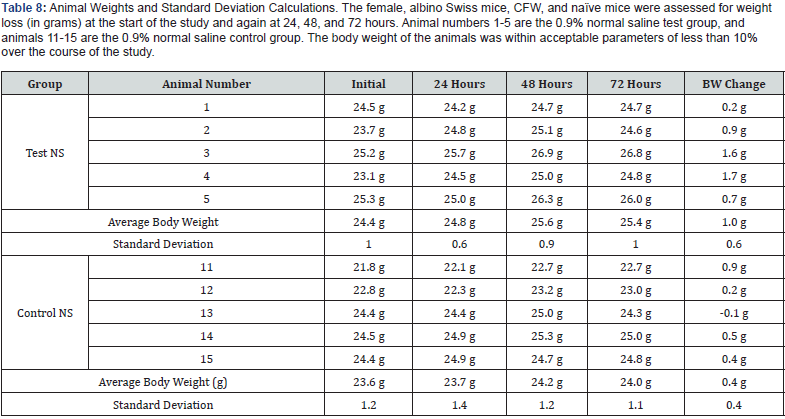
Two groups of five female, albino Swiss mice (Mus musculus), CFW, and naïve mice (Charles River Laboratories) at approximately 5 - 6 weeks old, were injected with either the test article extract or the corresponding control vehicle. Mice were weighed prior to treatment to the nearest 0.1g, treated intravenously with either control or test material, and returned to their housing. At the start of the study, all mice weights were between 21.8 and 25.3 grams, falling within ± 20% of the mean body weight. After treatment, the mice were observed for mortality and signs of pharmacological and/or toxicological effects at 4 ± 0.75. 24 ± 2, 48 ± 2 and 72 ± 2 hours post-injection.
Evaluation Criteria
According to ISO guidelines, the test is considered negative if none of the animals injected with the test article extract show a significantly greater biological reaction than the animals treated with the control vehicle extract. Death in two or more mice or other toxic signs such as convulsions, prostration, or body weight loss greater than 10% in three or more mice are interpreted as significant biological reactions.
Bacterial Mutagenicity Test-Ames Assay
This assay was performed in compliance with the following international standard: ISO 10993-3:2003.
Test Article Extraction
This assay was performed in compliance with the following international standards: ISO 10993-3:2003.
For extraction, the test article was placed in sterile vessels and prepared at a ratio of 120cm2 to 20 mL of extraction vehicle (0.9% Normal Saline only). The extraction mixtures and corresponding control blanks were incubated for 72 ± 2 hours at 37 ± 1 °C. At the start of the extraction, the solutions appeared clear and free of particulates. The ex-tracts were agitated during and after extraction, and the liquid was aseptically decanted. The test article was observed after all extractions to be intact with no macroscopically observable degradation, and the extracts were particulate-free, clear, and normal in color. After decanting, the unfiltered extracts were used immediately.
Control substances were prepared and used in the mutagenicity assay as described in Table 1.
Negative (Vehicle) Control
Tester strains were plated with the saline extraction blanks at the corresponding maximum concentration, with and without S9. These plates were negative controls and provided background lawn and revertant colony formation.
Test Method
The working cultures of the tester strains were prepared from frozen working stocks. To create working cultures for each bacterial strain used in the assay, approximately 30μL was transferred from the frozen working stock into 40 mL of Oxoid nutrient broth and incubated, with shaking, at 37 ± 2 °C until an optical density (at 650 nm) of 0.9 – 1.3 was reached (See Table 2). This culture was used for the mutagenicity test and genotypic confirmation.
For the mutagenicity test, a top agar consisting of 0.6% Difeo agar in 0.5% NaCl was melted and a solution of 0.5 mM L-histidine/0.5mM biotin or 0.5mM L-tryptophan was added to the melted top agar at a ratio of 10 mL per 100 mL agar, as required. The supplemented agar was aliquoted, 2 mL per tube and held at 45 ± 2 °C. To prepare the top agar for treatment, 0.1 mL of the test article or control, 0.1 mL of the bacteria culture and 0.5mL of PBS were added to the molten agar. The mixture was briefly vortexed and poured onto a room temperature minimal glucose agar plate (1.5% Difco agar, 0.4 - 2% glucose, in Vogel- Bonner medium E). Metabolic activation was provided by adding 0.5 mL of the S9 mix instead of the PBS. The plates were allowed to harden and then incubated for 48 - 72 hours at 37 ± 2 °C. All plates were counted using an automatic image analysis system. Negative control and test article treated plates were also examined for the presence of a bacterial lawn.
Exogenous Metabolic Activation
The in vitro metabolic activation system used in this assay was comprised of Sprague Dawley rat liver enzymes and a cofactor pool. The enzymes are contained in a preparation of liver microsomes (S9 fraction) from rats treated with Arochlor to induce the production of enzymes capable of transforming chemicals to more active forms. Immediately prior to use, the S9 was thawed and mixed with a cofactor pool to contain 5% microsomal enzymes, 5mM glucose 6-phosphate, 4mM -nicotine-adenine dinucleotide phosphate, 8 mM magnesium dichloride (MgCl2) and 33mM potassium chloride (KCI) in a 200mM phosphate buffer at pH 7.4. The S9 was purchased from Moltox (Boone, NC) and maintained frozen at less than -70 °C.
Intracutaneous Irritation Test
This assay was performed in compliance with the following international standards: ISO 10993-10:2010. This study used approximately 12-week-old male albino rabbits (Oryctolagus cuniculus) New Zealand White strain (Bakkom Rabbitry). The animals were supplied with certified commercial feed, ad libitum, and weighing between 2.4 and 3.2 kg at the experimental start of the study. There were no known contaminants present in the feed expected to interfere with the test results.
Test Article Extraction
The test article was extracted intact, placed into test tubes, and prepared at a ratio of 120cm2 to 20mL of extraction vehicle (0.9% normal Saline (NS), Braun). The extraction mixtures and corresponding control blanks were incubated for 72 ± 2 hours at 37 ± 1 °C. At the start of the extraction, the solutions appeared clear and free of particulates. At the end of the extraction period, the vessels were shaken well, and the liquid aseptically decanted into a sterile vessel. The test article was observed after all extractions to be intact with no macroscopically observable degradation and the extracts were normal in color, clear in transparency, and free of particulates. After decanting, the unfiltered extracts were maintained at room temperature and used within 24 hours of preparation.
Test Method
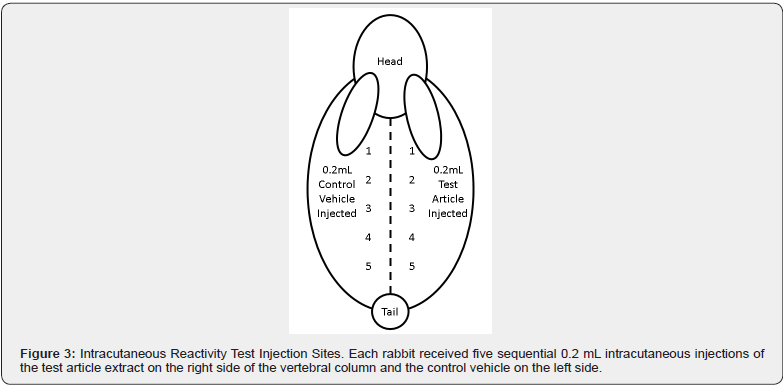
The weight of each animal was recorded prior to the test injection. The fur of the animals was clipped on both sides of the spinal column to expose a sufficient sized area for injection. Five intracutaneous injections of the test article extract and vehicle control were administered to each rabbit (See Figure 3).
Observations and Scoring
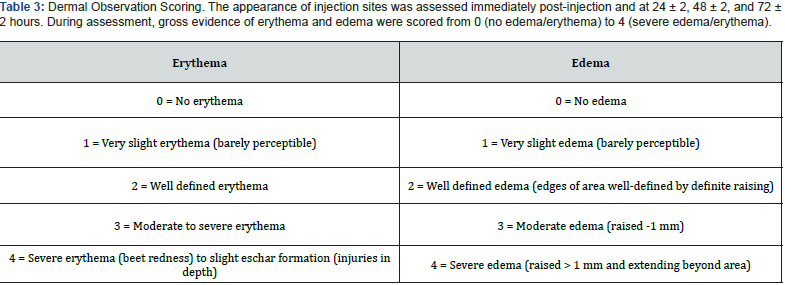
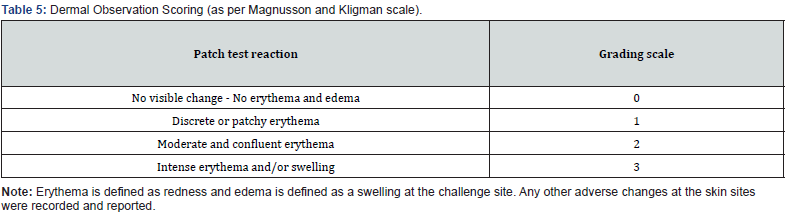
The animals were observed daily for abnormal clinical signs. The appearance of each injection site was noted immediately post injection and at 24 ± 2, 48 ± 2, and 72 ± 2 hours. The tissue reactions were rated for gross evidence of erythema and edema (Table 3 was used to score the reactions). According to the test protocol, if the difference between the average scores for the test article and the vehicle control is ≤ 1.0, the test article is not considered an intracutaneous irritant.
Maximization Sensitization Test
This assay was performed in compliance with the following international standards: ISO 10993-10:2010.
Seventeen male albino guinea pigs (Cavia porcellus), Hartley strain (specific pathogen free, Charles River Laboratories) was used for this study. All animals were approximately 5 weeks old and weighed from 307.3 to 361.5 g at the initiation of the study.
Test Material Preparation
For the first and second induction and challenge extractions, the test article remained intact, was placed into test tubes and extracted at a ratio of 120cm2 to 20mL of extraction vehicle (0.9% Normal Saline only, Braun). The extraction mixtures and corresponding control blanks were incubated for 72 ± 2 hours at 37 ± 1°C. At the start of the extraction, the solutions were manually agitated and appeared clear and free of particulates. The extracts were agitated during and after the extraction period, and the liquid was aseptically decanted into a sterile vessel. The test article was observed after all extractions to be intact with no macroscopically observable degradation, clear in transparency, free from particulates, and normal in color. After decanting, the unfiltered extracts were maintained at room temperature and used within 24 hours of preparation. Other test reagents used were Freund’s Complete Adjuvant (FCA, Difco), 10% Sodium Lauryl Sulfate (SLS) in Mineral Oil (Wu Xi AppTec) to enhance the potential of weak sensitizing agents.
Test Article Administration
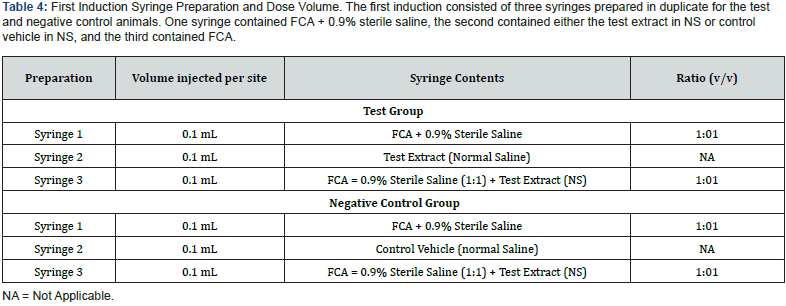
First Induction / Intradermal Injection: Three syringes were prepared for the test animals and three for the negative control animals as indicated in Table 4. The prepared syringes were injected in pairs on each side of the dorsal mid-line. The six injection sites were within a 2cm x 4cm area. Injections that included FCA were made slightly deeper to minimize adverse tissue sloughing.
Second Induction / Topical Application: On day 6, the injection site area was clipped free of fur and treated with 0.5mL of 10% (w/w) SLS prepared by mixing solid SLS with mineral oil. The day following the SLS treatment, the remaining SLS residue was gently wiped from the injection site area with gauze.
On day 7, the test article extracts (0.3mL) were applied to a 2cm x 4cm piece of filter paper (Whatman) to saturation and applied after SLS removal. The patch was secured to the site with non-permeable tape on the test animals and the trunk wrapped with elastic bandage and Transpore® tape. The control animals received a similar patch with the control vehicles. Freshly prepared extracts were used for this administration and were removed after 48 ± 2 hours of application.
Challenge Patch / Topical Application: Fourteen days after the topical induction phase, the challenge procedure was initiated on the eleven test animals and six negative control animals. A 2cm x 2cm filter paper patch was saturated with 0.3mL of freshly prepared test article extract and applied to each test animal’s fur clipped right flank. A 2cm x 2cm filter paper patch was saturated with 0.3 mL of freshly prepared control vehicle and applied to each animal’s fur clipped left flank.
The negative control animals were challenged identically with similarly prepared patches. The left side of each animal was patched with a filter paper patch saturated with 0.3 mL of the control vehicle. The right side was patched with a filter paper patch saturated with 0.3 mL of the prepared test article extract applied to the fur-clipped flank. The trunk of each animal was wrapped for 24 ± 2 hours with an expandable wrapping material and secured with tape.
Observations and Scoring
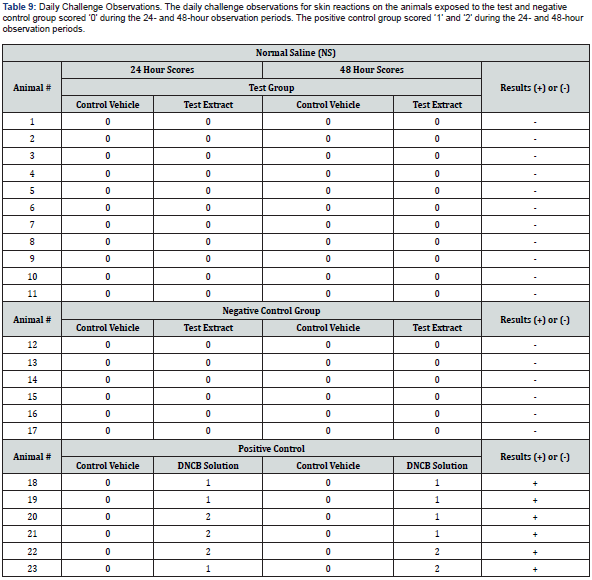
The following day (24 ± 2 hours) after challenge exposure, the patches were re-moved, and the site was wiped gently with a gauze sponge soaked in 70% isopropyl alcohol prior to each scoring period. The challenge sites were observed for irritation and sensitization reaction, as indicated by erythema and edema. Daily challenge observation scores were recorded 24 ± 2 and 48 ± 2 hours after patch removal in accordance with the classification system for skin reactions in Table 5. Daily animal health observations were recorded throughout the study period.
Results
Analysis of Residual Glutaraldehyde in an Amnion Membrane by HPLC-MS
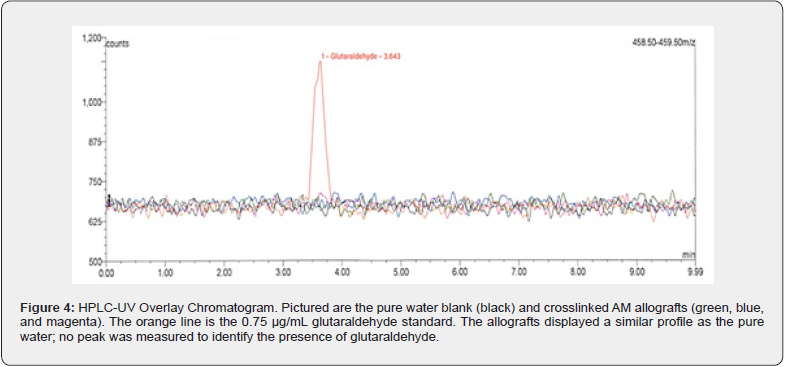
All LC-MS system suitability results were reported as acceptable. The coefficient of determination (r2) for glutaraldehyde was 0.99, and the instrument’s precision was confirmed to be 2.4%. The check standard accuracy was reported at 96.7%. In all test article extracts assayed (n = 3), no residual glutaraldehyde was detected (Figure 4).
Cytotoxicity Using L-929 Mouse Fibroblast Cells
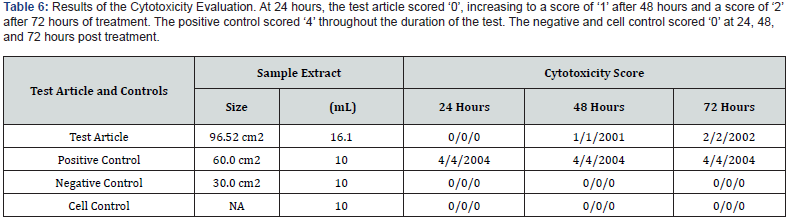
The negative and cell control were considered ‘0’, as the cells did not display a cytotoxic response. The positive control displayed a severe cytotoxic reaction, resulting in a score of ‘4’. Therefore, the test system responded normally and met the criteria for a valid assay (See Table 6). In this assay, the test article did not induce cytotoxicity. The test article scored ‘0’ at 24, ‘1’ at 48, and ‘2’ at 72 ± 4 hours and is considered non-cytotoxic under the conditions of this test.
Acute Systemic Injection Test
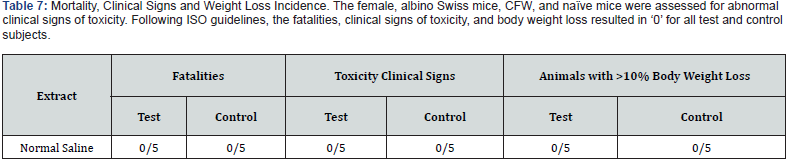
None of the animals in this study were observed with abnormal clinical signs indicative of toxicity during the 72-hour test period. All animals were alive at the end of the 72-hour test and body weight loss was within acceptable parameters throughout the study (See Table 7). The vehicle control-treated animals showed no signs of toxicity at any of the observation periods, and no animals lost more than 10% of their body weight, indicating the test to be valid (See Table 8). These findings suggest that the test article has met the ISO Acute Systemic Injection Test requirements. The GA crosslinked AM did not cause a toxic reaction.
Bacterial Mutagenicity Test-Ames Assay
In this assay, the test article did not induce substantial increases in reversion rates of the type associated with mutagenesis. Furthermore, no substantial test article toxicity was noted that may have interfered with the ability of the test system to detect mutagens. As none of the tester strains showed an increase in reversion rates when treated with the test article, the test article is determined not to have caused an increase in point mutations, exchanges, or deletions. Based on the criteria and conditions of the study protocol, the test article is considered non-mutagenic.
Intracutaneous Irritation Test
After the 72 ± 2 hours observation period, all erythema grades plus edema grades at 24 ± 2, 48 ± 2, and 72 ± 2 hours were totaled separately for each test sample or control for each animal. To calculate the score of a test sample or control on each animal, each total was divided by 15 (three scoring time points times five test or control sample injection sites). To determine the overall mean score for each test sample and each corresponding control, the score was added for the three animals and divided into three. The final test sample score was obtained by subtracting the score of the control from the test sample score.
Crosslinked AM allografts were not found to be an intracutaneous irritant by the intracutaneous irritation assay. Comparative results (average test article extract scores – average control article scores) were equal to 0.2, less than 1.0, which was the cut-off for this test. None of the animals showed abnormal clinical signs and no significant dermal reactions were observed during the 24-, 48-, and 72-hour observation periods.
Maximization Sensitization Test
Daily challenge observations for skin reactions demonstrated that none of the animals exposed to test article extract exhibited erythema or edema at the challenge sites and were scored a ‘0’ during the 24- and 48- hour observation periods The negative control group animals exhibited no erythema or edema at the challenge sites, therefore grades of ‘0’ were observed during the 24- and 48- hour observation periods. In the positive control group, sensitization reactions were observed with discrete or patchy erythema scoring ‘1’ and moderate and confluent erythema scoring ‘2’ during the 24- and 48-hour observation period (See Table 8). None of the animals involved in the study exhibited any abnormal clinical signs during the entirety of the study period. Under the conditions of this test, the results demonstrated that crosslinked AM allografts were not found to elicit a sensitization response greater than ‘0’.
Discussion
It is not common practice in a research lab to evaluate products using ISO standards. This extensive testing has been left to medical device manufactures when pre-paring documentation to submit to the FDA. With the FDA moving towards acceptance of the ISO evaluations, standards should become well understood, and the evaluations should be available in the literature. One argument for not performing and reporting these evaluations is that the results are qualitative with little to no quantitative evaluations. Given this argument, these standards are accepted by nearly all medical device regulatory bodies. Worldwide, these standards have been established by the International Organization for Standardization to regulate a new product’s safety, operational efficiency, environmental performance, reliability, and quality [25]. In this study the biocompatibility of AM treated with a crosslinker, glutaraldehyde, was assessed by a series of tests designed to evaluate its performance per ISO guidelines and determine its safe application as a potential biologically derived, chemically cross-linked, medical device.
The AMs were generally easy to work with, allowing all test extracts to be readily evaluated. The initial experiment, while not an ISO evaluation, was used to determine if any residual GA, a cytotoxic substance, was present in the allografts post processing. In the bloodstream, glutaraldehyde is broken down and expelled by exhaling as carbon dioxide. Nevertheless, GA is a skin, eye, and nasal irritant causative of more severe side effects like bronchitis, wheezing, and asthma [21,24]. The test articles and controls were analyzed by HPLC-MS to accomplish the initial experiment, resulting in no detectable glutaraldehyde in any of the test extracts. Therefore, it can be concluded that the GA introduced as a crosslinker in the processing of AM has reacted entirely, and any residual GA has been quenched or washed during processing.
To further evaluate the GA treated AM, extracts of the allografts were used to determine if there were cytotoxic effects on L-929 cells in culture, compared to a positive and negative control. The extracts were considered non-cytotoxic under the conditions of this study. In general, GA is one of the most widely used chemical crosslinking agents used for collagen scaffolds [26,27]. However, in recent years it has fallen out of favor in tissue engineering due to local cytotoxic effects [27]. Conversely, other studies on AM and their byproducts demonstrate that AM protein extracts can inhibit the metabolic activity of cancerous cell lines like hepatocellular carcinoma, inducing selective cyto-toxicity and cell death [28,29]. In general, however, AM membranes are not cytotoxic [30] and in the current evaluation GA-treated membranes are not cytotoxic.
In the intracutaneous irritation test, three albino rabbits received five sequential 0.2 mL intracutaneous injections of the test article extract on the right side of the vertebral column and the control vehicle on the left side of the vertebral column. Next, the irritation reaction of the test article solutions were compared to the vehicle controls and recorded over a 72-hours according to the standard ISO irritation scoring system. The test criteria implemented by ISO 10993-10 states that if the difference between the average scores for the extract of the test article and vehicle control is less than or equal to 1.0, the test article is considered to have met the test requirements. The results demonstrated that the test article met the ISO 10993-10 requirements, and no significant dermal reactions were observed. These results reveal amniotic membranes crosslinked with glutaraldehyde to be safe and do not elicit a skin reaction.
When selecting a new material for human contact in medical applications, it is essential to ensure that it will not stimulate the immune system, resulting in an allergic reaction. An immune response can occur if substances leach out of the material. Therefore, a guinea pig maximization sensitization test was conducted to determine the test article’s allergenic potential or sensitizing capacity. Eleven guinea pigs were assessed for a sensitization response, based upon the dermal scores. Additionally, results were based on the percentage of animals exhibiting a sensitization response. The use of FCA and SLS was required to enhance the potential of weak sensitizing agents. The evaluation criteria used for dermal observation scoring (See Table 5) was followed to establish grades of ‘1’ or greater in the test group to indicate sensitization, provided grades of less than ‘1’ are observed on the control animals. If grades of ‘1’ or greater are noted on control animals, then the reactions of the test animals that exceed the most severe control reaction were presumed to be due to sensitization. Moreover, the test is considered invalid if less than ten test animals or less than five control animals survived the duration of the study or if any animals developed frank infection at injection sites. The tests concluded that no animals challenged with the test article extract or in the negative control group showed a sensitization response greater than ‘0’ during daily challenge observations. In contrast, the positive control group animals scoring ‘1’ and ‘2’ sensitization reactions were observed during the 24- and 48-hour observation period.
Chemical cross-linking agents have been known to be toxic [26]. GA is a chemical cross-linker that can induce greater toxicity [31]. Since it has been established that GA can cause toxic reactions, two different toxicity evaluations were performed on the glutaraldehyde-treated AM: acute systemic toxicity and genotoxicity. The ISO does not call for these evaluations for prolonged surface contacting devices. These experiments were added to evaluate potential toxic reactions created by adding the GA to the AM. Both tests resulted in determining the allografts to be not toxic.
Conclusions
Despite a century-long history of clinical use, little improvement has been made to amniotic tissue as a wound care modality. This work describes a cross-linking technology used to increase the handling characteristics, durability and possibly the re-sorption rate of amniotic tissue allografts, for the first time, reports the ISO biocompatibility evaluations of such products. Further studies in cross-linked human amnionderived allografts using glutaraldehyde will provide insight into innovative applications as a potential wound-healing agent and thus improving wound management.
The biocompatibility testing performed in this study indicates all test article ex-tracts crosslinked with GA to be negative for residual glutaraldehyde, cytotoxicity, biological response, mutagenesis, and causing intracutaneous irritation. The requirements for product safety set by the International Organization for Standardization have been met. Ultimately, these results demonstrate glutaraldehyde-treated amniotic membrane allografts are safe for use as a medical device.
References
- gov.
- Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, et al. (2008) Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J 95(8): 4013-4024.
- Vo A, Diller R, Kellar R (2017) Characterization and clinical applications of amniotic membranes. J Pharmacol Clin Res 4(4): 555645.
- Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, et al. (2012) Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res 349(2): 447-458.
- Gholipourmalekabadi M, Farhadihosseinabadi B, Faraji M, Nourani MR (2020) How preparation and preservation procedures affect the properties of amniotic membrane? How safe are the procedures? Burns 46(6): 1254-1271.
- Kadkhoda Z, Tavakoli A, Rafiei SC, Zolfaghari F, Akbari S (2020) Effect of amniotic membrane dressing on pain and healing of palatal donor site: a randomized controlled trial. Int J Organ Transplant Med 11(2): 55-62.
- Rama P, Giannini R, Bruni A, Gatto C, Tiso R, et al. (2001) Further evaluation of amniotic membrane banking for transplantation in ocular surface diseases. Cell Tissue Bank 2(3): 155-163.
- Rocha SCM, Baptista CJM (2015) Biochemical properties of amniotic membrane. Amniotic Membrane: Origin, Characterization and Medical Applications 19-40.
- Abul A, Karam M, Rahman S (2020) Human amniotic membrane: A new option for graft donor sites–Systematic review and meta‐analysis. Int Wound J 17(3): 547-554.
- Ricci E, Vanosi G, Lindenmair A, Hennerbichler S, Peterbauer-SA, et al. (2013) Anti-fibrotic effects of fresh and cryopreserved human amniotic membrane in a rat liver fibrosis model. Cell and Tissue Banking 14(3): 475-488.
- Chen W, Yang S, Li S, Lang JC, Mao C, et al. (2019) Self-assembled peptide nanofibers display natural antimicrobial peptides to selectively kill bacteria without compromising cytocompatibility. ACS Appl Mater Interfaces 11(32): 28681-28689.
- Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, et al. (2004) Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation 78(10): 1439-1448.
- Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, Van Griensven M, et al. (2007) Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng 13(6): 1173-1183.
- Leal‐Marin S, Kern T, Hofmann N, Pogozhykh O, Framme C, et al. (2021) Human Amniotic Membrane: A review on tissue engineering, application, and storage. J Biomed Materials Res Part B Appl Biomater 109(8): 1198-1215.
- Yazdanpanah G, Paeini-Vayghan G, Asadi S, Niknejad H (2015) The effects of cryopreservation on angiogenesis modulation activity of human amniotic membrane. Cryobiology 71(3): 413-418.
- O'brien FJ (2011) Biomaterials & scaffolds for tissue engineering. Materials Today 14(3): 88-95.
- Gunasekaran D, Thada R, Jeyakumar GFS, Manimegalai NP, Shanmugam G, et al. (2020) Physicochemical characterization and self-assembly of human amniotic membrane and umbilical cord collagen: A comparative study. Int J Biological Macromolecul 165: 2920-2933.
- Olde Damink LHH, Dijkstra PJ, Van Luyn MJA, Van Wachem PB, Nieuwenhuis P, et al. (1995) Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J Material Sci Mater Med 6: 460-472.
- Sarvari R, Keyhanvar P, Agbolaghi S, Roshangar L, Bahremani E, et al. (2022) A comprehensive review on methods for promotion of mechanical features and biodegradation rate in amniotic membrane scaffolds. J Material Sci 33(3): 32.
- Zeiger E, Gollapudi B, Spencer P (2005) Genetic toxicity and carcinogenicity studies of glutaraldehyde-a review. Mutation Research/Reviews in Mutation Res 589(2): 136-151.
- Wilbur SB, Harper C, Demchuk E, Ingber SZ, Wohlers D, et al. (2017) Toxicological profile for glutaraldehyde.
- Scheffel DLS, Soares DG, Basso FG, de Souza Costa C, Pashley D, et al. (2015) Transdentinal cytotoxicity of glutaraldehyde on odontoblast-like cells. J Dent 43(8): 997-1006.
- Wijaya RI, Ilyas M (2021) Allergic Contact Dermatitis Among Healthcare Workers Exposed to Glutaraldehyde. The Indonesian J Community Occupational Med 1(2): 94-100.
- Abdel-Hamid MA, Abbas D, Elokda EE, El-Gwaily MS (2019) Adverse health effects of glutaraldehyde and its relation to workplace safety measures and work practices of health care workers. Egyptian J Occupational Med 43(2): 297-312.
- International Organization for Standardization. Standards (2019) ISO.
- Adamiak K, Sionkowska A (2020) Current methods of collagen cross-linking. Int J Biological Macromolecul 161: 550-560.
- Ren Y, Fan L, Alkildani S, Liu L, Emmert S, et al. (2022) Barrier membranes for guided bone regeneration (gbr): A focus on recent advances in collagen membranes. Int J Molecular Sci 23(23): 14987.
- Mamede AC, Guerra S, Laranjo M, Carvalho MJ, Oliveira RC, et al. (2015) Selective cytotoxicity and cell death induced by human amniotic membrane in hepatocellular carcinoma. Med Oncology 32(12): 1-11.
- Mamede AC, Laranjo M, Carvalho MJ, Abrantes AM, Pires AS, et al. (2014) Effect of amniotic membrane proteins in human cancer cell lines: an exploratory study. J Membrane Biol 247(4): 357-360.
- Gholipourmalekabadi M, Bandehpour M, Mozafari M, Hashemi A, Ghanbarian H, et al. (2015) Decellularized human amniotic membrane: more is needed for an efficient dressing for protection of burns against antibiotic-resistant bacteria isolated from burn patients. Burns 41(7): 1488-1497.
- Islam MM, AbuSamra DB, Chivu A, Argüeso P, Dohlman CH, et al. (2021) Optimization of collagen chemical crosslinking to restore biocompatibility of tissue-engineered scaffolds. Pharmaceutics 13(6): 832.






























