Hepatocurative and Gluco-stabilizing Potentials of Ethanol Extract of Stem bark of Flacourtia indica in Aluminium Chloride induced Toxicity in Albino Wistar rats
Idoko A1*, Emmanuel UG1, Yakubu OE3, Ugwudike PO1 and Patricia NA2
1 Department of Biochemistry, Caritas University, Nigeria
2Department of Microbiology, University of Ibadan, Nigeria
3 Department of Biochemistry, Federal University Wukari, Nigeria
Submission: November 22, 2018;Published: January 17, 2019
*Corresponding author: Idoko A, Department of Biochemistry, Faculty of Natural Sciences, Caritas University, Amorji – Nike, P.M.B. 01784, Enugu, Nigeria
How to cite this article: Idoko A, Emmanuel U, Yakubu O, Ugwudike P, Patricia N. Hepatocurative and Gluco-stabilizing Potentials of Ethanol Extract of Stem bark of Flacourtia indica in Aluminium Chloride induced Toxicity in Albino Wistar rats. Curr Trends Biomedical Eng & Biosci. 2019; 17(5): 555971. DOI: 10.19080/CTBEB.2019.17.555971.
Abstract
Herbal therapies have been used to manage liver diseases resulting from hepatotoxicity and hyperglycemia. The study investigated the hepatoprotective and gluco-stabilizing abilities of ethanol extracts of stem bark of Flacourtia indica in albino Wistar rats. Thirty-one rats of mixed sex, weighing 165-285g were used and divided into five groups of A to E. Acute toxicity study of the plant’s stem bark was conducted on group E (20 rats). At phase 1, group A (negative control) was made up of 3 rats administered no AlCl3 and no leaf extracts while groups B (positive control), C and D (test) made of 4 rats each, administered 260mg/kg body weight AlCl3 only, for 7 days. At phase 2, groups C and D were treated with stem bark extract of Flacourtia indica for 7 days. Activities of Alanine aminotransferase, Aspartate aminotransferase, Alkaline phosphatase, concentrations of Bilirubin, Albumin, Total protein and blood glucose were assayed with histopathological study on euthanized rats’ liver.
Results of phase 1 showed significant (p˂0.05) increase in the liver function enzyme and blood glucose after induction with AlCl3 compared with (phase 2) values of liver function enzyme and blood glucose with significant (p˂0.05) decrease after treatment with stem bark extract. Histopathology results in phase2 showed regeneration and healing of damaged hepatocytes of phase1. In conclusion, the liver injury induced by AlCl3 was found to be effectively managed by the treatment with Flacourtia indica’s ethanol stem bark extracts, with blood glucose stabilized. This could be as a result of the antioxidants and phytochemical contents of the plant, with diver’s potency to scavenge free radicals and reactive oxygen species.
Keywords: Flacourtia indica; Gluco-stabilizer; Hepatic function; Hepatoprotective; Hyperglycemia; Stem bark
Abbrevations: ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; WHO: World Health Organization; BIL: Bilirubin; TP: Total Protein
Introduction
The liver, a reddish – brown heaviest and largest internal organ weighs about 1.5 – 1.6 kg in an adult male and is about the size of an American football [1]. It is located behind the ribcage on the upper right side of the abdomen and is divided into four lobes with multiple lobes containing hepatic cells (hepatocytes). A normal liver enjoys an adequate blood supply and ability to regenerate its own tissues within few days [2]. The liver is known to perform over 500 functions in the body which include detoxification, excretion, digestion, metabolism, storage and homeostasis. It detoxifies harmful substances that are toxic to the body such as drugs, chemicals, heavy metals, alcohol and pesticides [3]. The liver excretes toxic chemical substances in the bile as well as xenobiotics, hormones and other by-products [4]. The liver plays a major role in homoeostasis stability of trace elements [5]. The liver is a major store house of glucose (in form of glycogen), iron and vitamins such as vitamins A, folate (B9), B12 and D [6]. Enzymes such as alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and hormones are synthesized by the liver [7]. However, the liver is adversely affected by overloaded toxic substances, resulting in hepatotoxicity [8].
Hepatotoxicity is implicated with chemical induced liver damage resulting in chronic and acute liver diseases. Over 800 chemicals causing hepatic injuries abounds. They include drugs used to suppress pain and reduce inflammation such as ibuprofen, acetaminophen or paracetamol, naproxen and aspirin (Non-Steroidal Anti-inflammatory Drugs), alcohol, industrial and laboratory chemicals such as Aluminum trichloride AlCl3 and carbon tetrachloride CCl4 and natural toxins such viruses, aspergillus flavus [6,9,10]. Meanwhile, AlCl3 has demonstrated high potency in exerting hepatotocity, the mechanism by which AlCl3 exert its injury on the liver is not yet clear [9]. These induced toxic chemicals in the hepatic tissue, render the liver disease with malfunction including hepatitis, liver cancers, fatty liver, primary biliary cirrhosis, primary sclerosing cholangitis, hemochromatosis, hyperoxaluria and oxalosis and Wilson’s disease [11,12]. The earth is made up of about 8.13% of aluminium. The metal has got wide utilization, therefore increasing the chances of exposure into the body through the intestinal tract and the lung. It is a used in manufacturing cosmetics such as roll-on and deodorant, cooking utensils, food additives and drugs such as antacids. In water purification, aluminium salts are also used [13].
The practice of traditional medicine, using plants and plants products for the treatment of ailments is an age long practice. Several medicinal plants with their herbal functions have been discovered and more are still be researched on [14]. Medicinal plants have been found to possess several activities such as hepatoprotective, nephroprotective, antimicrobial, anticancer, anti-inflammatory, hypoglycemic, antihypercholesterolemia, hypoglycemia, heamatinic and antioxidant activity [15-17]. These beneficial therapeutic properties and efficient pharmacological impacts of medicinal plants on man and animals are not farfetched from the numerous bioactive compounds they contain [18]. The availability of herbal alternatives for the management of liver disease and other ailments is been encourage by world health organization (WHO) to be better developed for improved efficacy, safety of use, cheap to purchase and accessible for patronage [19].
Flacourtia india is a wide spread medicinal plant, of Salicaceae family, tribe of Flacourtiaceae, genus of Flacourtia and specie of Indica [20, 21]. It is known as Governor’s plum in English, kondai or Katai in India, Cilimu in China and in Nigeria: it is called Akpuru in Igbo, Isada in Hausa and Iyeye in Yuroba [22]. The plant is tropical specie with a natural geographical occurrence in Africa and Asia. Predominantly, Flacourtia india is said to be a native to both Africa and East Asia [23]. Flacourtia indica is a bushy small shrub like tree, having a spiny, erect, rough, strong stem bark, that extend with branches. It spines branched and spread up to 12cm long and to a maximum height of about 15cm (50 feet) [22]. Several pharmacological abilities of Flacourtia indica have been exploited with the whole plant, leaf, seed, fruit, stem, bark and root. These include hepatoprotection against AlCl3, paracetamol and CCl4 induced hepatic damage [9,24], anti-diabetic, anti-anxiety, antimicrobial, anti-malaria, anti-asthmatic, diuretic, antioxidant, analgesic/anti-inflammatory and anti-hyperlipidemia [18,25- 27]. The plant has been reported by previous researchers in their phytochemical analysis to contain several bioactive compounds, including phenolic compounds, flavonoids, saponins, carbohydrate, Coumarin Glycosides [28].
Blood glucose concentration is an important index handled by the liver in monitoring and stabilizing blood glucose levels. In post absorptive state, the fasting blood glucose concentration of a healthy (normal) individual is 70 – 100mg/dl (4.5-5.5mmol/l). However, after eating a carbohydrate meal, the level of blood glucose may rise to 120-140mg/dl. It is generally established that the value for plasma concentration is about 15% slightly higher than the value of blood glucose. Hypoglycemia implies a decrease in blood glucose concentration from the normal and hyperglycemia is an increase in blood glucose concentration from the normal [29,30]. Plants have been reported to have herbal ability in reducing high glucose concentration, not necessarily causing an unhealthy hypoglycemia, but attempting to stabilize blood glucose level in albino wistar rats [30]. Thus, in view of these, it became imperative to investigate the hepatocurative and gluco-stabilizing effects of stem bark of Flacourtia indica using ethanol solvent extraction in order to ascertain the previous claim and establish new fact.
Materials and Methods
Chemicals
Aluminium trichloride AlCl3 was purchased from BDH Laboratories/Chemicals Ltd, Poole, England. Kits used for the liver function assay were obtained from Randox Laboratories Ltd, 55 Diamond Road, Crumlin, country Antrim, BT29 4QY, United Kingdom. They include; Bilirubin (BIL), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Albumin (ALB), Total Protein (TP). All other chemicals used are pure and of analytical grade.
Collection and Preparation of Plant Samples
Fresh plant materials of Flacourtia indica (Governor’s plum) were collected from around Emene jurisdiction in Enugu state, Nigeria. The required plant stem bark was authenticated and a voucher number of PSB/109-12. A was given by Mr. Okafor, C.U., a botanist in plant tissue culture and biotechnology department, Faculty of Biological Science, University of Nigeria, Nsukka. The plant extract was prepared by pilling the bark from the stem of the plant and cut into pieces for easy air drying. The dried samples were ground into powder using an electric grinder. The powdered parts were soaked in ethanol and allowed to stay for 48 hours at room temperature after which they were filtered. After filtration, the samples were then taken and evaporated to dryness using water bath at 80oc. The evaporated extracts were reconstituted with distilled water relative to the weight of the evaporated extract. The volumes of the extracts to be administered were calculated according to the body weight of the rats using the formula:
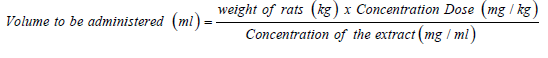
Collection and preparation of blood sample
Three milliliter (3mls) of blood was collected from the rats by capillary pressure insertion into the side of the eye using capillary tubes into a plain bottle, for the collection of serum used for biochemical assay (liver function test). The samples in bottles were stored at room temperature.
Acute Oral Toxicity (LD50) Study
Twenty (20) rats were used for oral acute toxicity study (LD50) of the plant. The acute toxicity study of ethanol stem bark extract of Flacourtia indica was evaluated in two phases as described by [31]. In the 1st phase, doses of 10mg/kg, 100mg/kg, and 1000mg/kg were administered to 3 rats each. The body weight of rat was noted before and after extract administration. Single dose of ethanol extract was administered orally and observed from the time of administration, for toxic symptoms, such as behavioral changes, loco-motion, convulsion and mortality, then overnight and finally for a time period of five days. In the absence of mortality in the first phase, higher doses of 1500mg/kg, 2500mg/ kg, 3500mg/kg and 5000mg/kg were then administered on 1 rat each of which one mortality was observed in the 2nd phase of the experiment at 3500mg/kg body weight. LD50, the amount or lethal dose of materials given all at once, which causes the death of 50% of a group of test animals was calculated with the formula below;
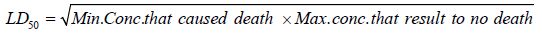
Study Animals
Albino Wistar rats of 165-285g weight, of either sex were obtained from university of Nigeria Nsukka. Animals were housed at an ambient temperature and relative humidity in the animals’ house of natural sciences, Caritas University, Amorji – Nike Enugu. The rats were allowed to acclimatize for one week prior to the experiment and had access to standardized pelletized finisher feed and clean water within the period the acclimatization. The principle of laboratory animals’ care and ethical guidelines for investigation of experimental pain in conscious animals were followed respectively [32,33].
Experimental Design
Experimental design was carried out in two phases thirty five (35) Wistar albino rats were used, divided into A, B, C, D and E as follows; at phase 1,
Group A: Control (negative control) consist of 4 rats, no Aluminum chloride (AlCl3 were administered.
Group B: Test control (positive control) consist of four (4) rats, were administered orally with 260mg of Aluminum chloride (AlCl3) without extract given.
Group C: Consist of four (4) rats, were orally administered with 260mg/kg body weight of rats.
Group D: Consist of four (4) rats were orally administered with 260mg/kg body weight of rats. At phase 2, groups C and D were treated with ethanol stem bark as follows;
Group C: Consist of four (3) rats, were treated with stem bark extracts of 500mg/kg body weight of rats.
Group D: Consist of four (3) rats were treated with stem bark extract of 700mg/kg body weight of rats.
At the end of phase 1 and 2, one rat from each group was randomly selected and sacrificed by euthanization using chloroform and liver removed for histopathology study.
Induction of Liver Injury
Liver injury was induced in rats of group B – D by single oral administration with 260mg/kg body weight of AlCl3 respectively.
Liver Function Assay
After collection of blood sample from rats serum was collected by clot retraction. Serum ALT, AST, ALP, Albumin, Total protein and Bilirubin were assayed with the use of kits from Randox Laboratories Ltd, 55 Diamond Road, crumlin, country Antrim, BT29 4QY, United Kingdom, following the manufacturer’s procedure.
Histopathological Studies
The selected models were sacrificed and dissected after which the liver organs were excised and fixed in a buffer medium of 0.9% of formalin solution in plain tissue bottles. The tissues were embedded in paraffin, solid section was cut 5um and stained with hemotoxin and eosin, the sections were examined and analyzed by the distant Specialist of histopathology, University of Nigeria under high microscopy instrumentation having photomicrographic attachment.
Statistical analysis
Results were expressed as mean ± standard deviation and analyzed using one-way ANOVA (analysis of variance, p value (p<0.05) was considered significant. A component of graph pad instat 3 software version 3.05 by graph pad Inc. was employed [34].
Results
Acute toxicity results
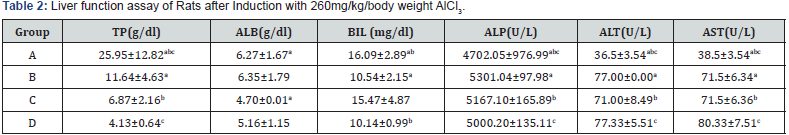
The oral administration of ethanol extract of F. indica stem bark resulted in behaviour changes and mortality up to the dose of 3500 mg/kg body weight at the second phase. Though, the LD50 of F. indica ethanol stem bark extract was calculated to be 4183mg/kg (higher than 3500 mg/kg). Table 1 shows the result of the acute toxicity in phase 1 and 2. The liver function test of rats after induction with 260mg AlCl3, for liver injury is shown in Table 2. A significant (p<0.05) increase was observed in TP, ALB, BIL, ALP, ALT and AST of control compared to test control and test groups.
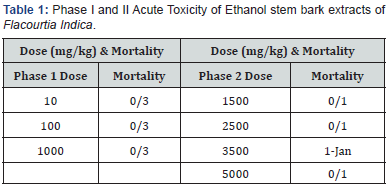
Table 3 shows liver function indices of rats administered various dose of stem bark (500mg and 700mg) extract of flacourtial- Indica for 0ne week. A significant (p<0.05) increase was observed in all parameters assayed in the test groups compared to the test control and control group. The significant increase (p<0.05) in Table 2 when compared to Table 1, reveals the potential hepato- healing effect of Flacourtia indica not necessarily in a dose dependent manner.
Results are mean ± standard deviation, Values in the same column bearing similar superscripts are significantly different at P<0.05. (n=4).
Key: A: Control Group; B: Test Control; C and D: Test groups.
TP: Total Protein; ALB: Albumin; BIL: Bilirubin; ALP: Alkaline Phosphatase; ALT: Alanine Transaminase; AST: Aspartate Transaminase
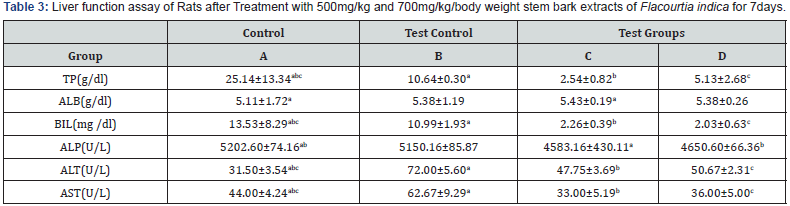
Results are mean ± standard deviation, Values in the same row bearing similar superscripts are significantly different at P<0.05. (n=4).
Key: A: Control Group; B: Test Control; C and D: Test groups; TP: Total Protein; ALB: Albumin; BIL: Bilirubin; ALP: Alkaline Phosphatase; ALT:
Alanine Transaminase; AST: Aspartate Transaminase
Table 4 presents the blood glucose concentration of rats after induction with AlCl3 and after Treatment with stem bark extracts of Flacourtia indica. After administration (treatment), with Flacourtia indica, the blood glucose concentrations of test groups (C and D) decreased significantly (p<0.05) compared to after induction with AlCl3 in a dose dependent pattern, thus acerbating induced hypoglycemia.
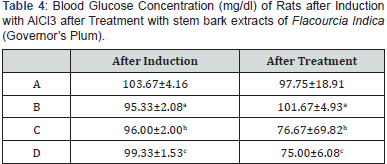
Results are mean ± standard deviation, Values in the same row bearing similar superscripts are significantly different at P<0.05. (n=4).
Key: A: Control Group; B: Test Control; C and D: Test groups
Result of Histopathology Analysis
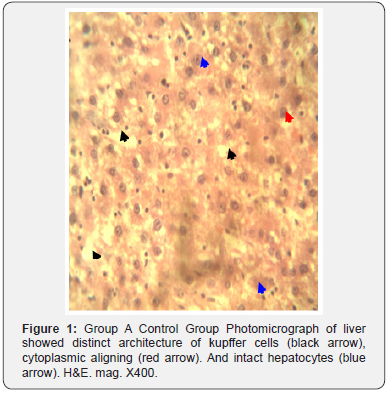
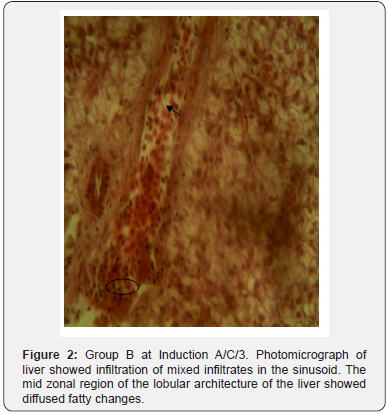
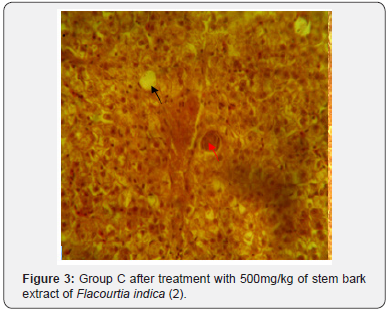
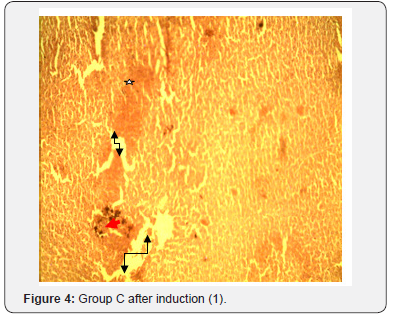
The results of histopathological study of liver tissue of rats at induction (group A and B), after induction with AlCl3 and after treatment with F. indica ethanol stem bark extract (group C and D) as cross sections are shown in Figure 1 & 2. Photomicrograph of liver showed cytoplasm containing empty (black arrow) and fluid filled (red arrow) appearing vacuoles which pushed out the nucleus and then formed a signet-ring like appearance. H&E. mag. 400X (Figure 3). Photomicrograph of liver showed minor distortion of the hepatic cords leading to enlarged sinusoids (black arrow), also macrophages (red arrow) and focal necrosis (star) were also observed. H&E. mag. 100X (Figure 4). Photomicrograph of liver showed massive influx of inflammatory cells (black arrow), damaged hepatocyte (red arrow), degeneration of the hepatocyte cords and the affected hepatocytes are aligned in deformed cords which compresses the lumen of the sinuses. Zonal fatty changes occurred in the liver lobules, diffusely affecting the same zone. The fatty degeneration, vacuoles appeared in the cytoplasm around the nucleus, because the lipid content may be dissolved in the course of embedding. The vacuoles are empty with absence of inflammatory cells (black arrows) and regenerated hepatocytes (white arrows). H&E. mag. 400X.
Discussion
The significant increase that was observed in the liver function indices in Table 2 of groups C and D, as compared to Table 3 was as a result of aluminium chloride administration. This result is consistent with other researchers’ who reported that aluminium chloride is capable of inducing hepatic injury (hepatotoxicity), using a minimal dose of 34 mg/kg body weight administered in rats’ diet [35] and oral exposure in drinking water of 40 male Wistar albino rats of 0, 64mg/kg, 128mg/kg and 256mg/kg body weight AlCl3 for 120days, respectively [9]. AlCl3 induced toxicity has been reported to have toxic effects on liver, kidney, biochemical dysfunction and general health challenge [13]. A significant increase was seen in blood glucose concentration of rats after induction with AlCl3 compared to after treatment with Flacourtia indica (Table 4). This increase could have resulted from the inability of the liver to secret insulin owing from the hepatic damage caused by AlCl3. The mechanism through which this was done was taught to be linked to hepatic-stress related release of epinephrine, a factor inhibiting insulin secretion [36].

The potent inducement of liver injury by AlCl3 was supported by histopathology results (Figures 1, 2, 4 & 5). The underlying mechanism through which AlCl3 induces hepatic damage, as hepatic toxicant, as shown by the histopathology results could be associated to fatty liver incidence as a result of accumulated triacylglycerols and lipoprotein [36]. The histopathological results’ examination of liver tissues reveals intact hepatocytes, distinct architecture of kupffer cells and cytoplasmic aligning of the negative control group. Induction by AlCl3 in test groups revealed several degrees of hepatic injuries including; infiltration of mixed infiltrates in the sinusoid, diffused fatty changes, focal necrosis, massive influx of inflammatory cells, damaged hepatocyte, degeneration of the hepatocyte cords and the affected hepatocytes are aligned in deformed cords which compresses the lumen of the sinuses (Figures 2, 4 & 5). Treatment with Flacourtia indica’s ethanol extract stem bark with 500 mg/kg and 700 mg/ kg in rat’s liver revealed regeneration of hepatocytes, absence of inflammation, with almost healed hepatic architecture (Figures 3 & 6). This is also consistent with [28], supporting the claim that Flacourtia indica protects the liver against AlCl3 hepatic damage in treated groups with healing features, typified by absence of damaged and degenerated hepatocytes, necrosis and inflammation.
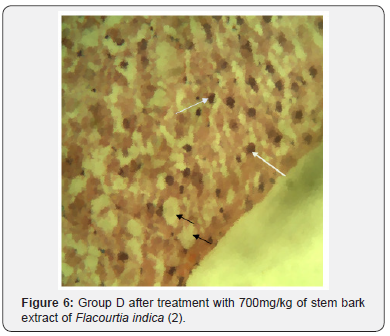
Treatment of the AlCl3 induced hepatotoxic rats with the stem bark extract of Flacourtia indica revealed a significant decrease in AST, ALT, BIL, TP, ALP and blood glucose concentration and a slight significant increase in serum albumin, implying that hepatic tissues are regenerating and healing. This observation was supported by the histopathology analysis of the liver tissues which shows recuperation of hepatic cells after administration of the stem bark extract. This is in support of the work of [28], who reported that Flacourtia indica prevents and protect AlCl3 and CCL4 induced rats’ hepatic damage through augmentation of antioxidant enzyme activity. Flacourtia indica appears to prevent the increase in the activities of the main liver function enzymes (ALP, AST, ALT, BIL and TP) by a counter and effective action against the hepatoxic damaging effects of AlCl3. This action might be due to Flacourtia indica’s potentials as an indirect antioxidant promoter and free radical scavenging abilities in preventing membrane failure and cellular necrosis [20].
Table 4 shows the blood glucose concentrations and effect of oral administration of the different doses after induction with 260 mg AlCl3 and 500 mg/kg and 700 mg/kg body weight of Flacourtia indica on blood glucose concentrations of rats. After administration of stem bark extract of Flacourtia indica, the blood glucose levels of test groups (C and D) decrease significantly (p<0.05) compared to after induction with AlCl3 in a dose dependent pattern, thereby bringing the concentration of blood glucose to a stabilized level from the AlCl3 induced hepatotoxic level. Reduction in blood glucose by Flacourtia indica’s stem bark could be due to the presence of both micro and macro mineral elements and phytochemicals, having antioxidant and blood glucose enzyme regulating and stabilizing control [19,28,37,38], reported the mineral elements composition in Flacourtia indica to include magnesium, manganese and zinc. Thus, the mechanism behind the stability of blood glucose by Flacourtia indica could be understood from the role of these mineral elements as cofactors to glycolytic enzyme especially hexokinase and phosphofructose [17]. The blood glucose level stabilizing effects of Flacourtia indica appears to be maintained by the constant furnishing of these mineral elements [37,38].
Conflict of Interests
Authors have declared that no competing interests exist.
References
- Alan F (2015) An overview of the liver: A series of fact sheets written in the field of liver disease. Hepatitis C Support Project 1: 1- 5.
- Enrich C, Pol A, Calvo M, Pons M, Jäckle ST (1999) Dissection of the multifunctional “receptor-recycling” endocytic compartment of hepatocytes. Hepatology 30(5): 1115-1120.
- Smedsrod B, Pertoft H, Gustafson ST, Laurent TC (1990) Scavenger functions of the liver endothelial cell. Biochem J 266(2): 313-327.
- Solaas K, Ulvestad A, Soreide O, Kase BF (2000) Subcellular organization of bile acid amidation in human liver: a key issue in regulating the biosynthesis of bile salts. J Lipid Res 41(7): 1154-1162.
- Lindner MC (1984) Other trace elements and the liver, Semin. Liver Dis 4(3): 264-276.
- Wilson GE, Davis RE (1983) Clinical chemistry of vitamin B6. Adv Clin Chem 23: 1-68.
- Pocker Y (2001) Bioinorganic and bioorganic studies of liver alcohol dehydrogenase. Chem Biol Interact 130(1-3): 383-393.
- Andrade RJ, Robles M, Fernández-Castañer A, López-Ortega S, López- Vega MC, et al. (2007) Assessment of drug-induced hepatotoxicity in clinical practice: a challenge for gastroenterologists. World J Gastroenterol 13(3): 329-340.
- Xu F, Liu Y, Zhao H, Yu K, Song M, Zhu Y, Li Y (2017) Aluminum chloride caused liver dysfunction and mitochondrial energy metabolism disorder in rat. J Inorg Biochem 174: 55-62.
- Riordan SM, Williams R (2002) Alcohol exposure and paracetamolinduced hepatotoxicity. Addict Biol 7(2): 191-206.
- Banankhah PS, Garnick KA, Greenblatt DJ (2016) Ketoconazole- Associated Liver Injury in Drug-Drug Interaction Studies in Healthy Volunteers. J Clin Pharmacol 56(10): 1196-1202.
- Keeffe EB, Friedman LM (2004) Handbook of liver diseases. (3rd edn.) Edinburgh: Churchill Livingstone. pp. 104-123.
- Lukyanenko LM, Skarabahatava AS, Slobozhanina EI, Kovaliova SA, Falcioni ML (2013) In vitro effect of AlCl3 on human erythrocytes: changes in membrane morphology and functionality. J Trace Elem Med Biol 27(2):160-167.
- Lewis HW, Elvin-Lewis MPH (1977) Medical Botany: Plants Affecting Man’s Health. John Wiley and Sons, New York. pp. 217-218.
- Kumar SV, Sanjeev T, Ajay S, Kumar SP, Anil S (2012) A Review on Hepatoprotective Activity of Medicinal Plants. IJARPB 2(1): 31-38.
- Idoko A, Ikpe VPO, Nelson NO, Effiong JU, Alhassan AJ, et al. (2017) Effects of Lime Juice and Honey on Lipid Profile of Cholesterol Enriched Diet Fed Rat Model. Annual Research & Review in Biology 20(3): 1-10.
- Idoko A, Ikpe VPO, Rita ON, Nelson NO, Effiong JU, et al. (2018) Hypoglycermic and Lipid Profile Lowering Effect of Chromolaena Odorata (linn) in Albino Wistar Rats Fed Different Concentrations of Cholesterol Enriched Diet. Universal Journal of Pharmaceutical Research 3(1): 37-42.
- Eramma N, Devaraja G (2013) Antibacterial potential and phytochemical analysis of Flacourtia indica (Burm.f.) Merr. root extract against human pathogens. Indo American Journal of Pharmaceutical Research 3(5): 3832- 3846.
- WHO (2014) WHO traditional medicine strategy 2002-2005. WHO: Switzerland.
- Gopi K, Karthikeyan M, Kannan M, Rajasekar R (2012) Flacourtia indica
- Sanjeeb K, Padmacharan B, Posa M, Sasmal D, Rarijan K, et al. (2013) Pharmacology Review of Flacourtia sepiara. Scholars of Academic Journal of Pharmacy 2(2): 89-93.
- Orwa C, Mutual A, Kindt R, Jamnadass R, Simons A (2009) Agroforestry database: a tree reference and selection guide. World Agroforestry Centre.
- Datta A, Rawat G (2008) Dispersal Modes and Spartial Patterns of Tree Species in Tropical Forests in Arunachal Pradesh, Northeast India. Tropical Conservation Science 1(3): 163-185.
- Marina Nazneen Md, Abdul MazidJoydev K, Kundu Sitesh C, Bachar FaridaBegum Bidyut KD (2009) Protective effects of Flacourtia indica aerial parts extracts against paracetamol-induced hepatotoxiciy in rats. Journal of Taibah University for Science 2: 1-6.
- Virendra S, Mahendar S, Smita S, Kori ML (2011) Antidiabetic effects of Flacourtia indica Merr in streptozotocin induced diabetic rats. Global Journal of Pharmacology 5(3): 147-152.
- Juthika K, Monika R, Sitesh CB, Kyung-Soo C, Joydeb KK (2013) Analgesic, anti-inflammatory, and diuretic activity of methanol extract of Flacourtia indica. Arch Bas App Med 1: 45-51.
- Satyanand T, Mahendra S, Dashrath S, Indu Y, Sunil S, et al. (2011) Anti- Asthmatic Potential of Flacourtia indica Merr. African Journal of Basic & Applied Sciences 3:201-204.
- Anindita B, Gangarao B (2016) Flacourtia indica (burm.f.) prevents ccl4 induced rat liver damage by augmenting antioxidant enzyme activity. European Journal of Pharmaceutical and Medical Research 3(4): 263- 270.
- Cornblath M, Hawdon JM, Williams AF, Aynsley-Green A, Ward-Platt MP, et al. (2000) Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics 105(5): 1141-1145.
- Yanai H, Adachi H, Katsuyama H, Moriyama S, Hamasaki H, et al. (2015) Causative anti-diabetic drugs and the underlying clinical factors for hypoglycemia in patients with diabetes. World J Diabetes 6(1): 30-36.
- Lorke D (1983) A New Approach to Practical Acute Toxicity Testing. Arch Toxicol 54(4): 275-287.
- National Institute of Health (1937) Guidelines for the Care and Use of Laboratory Animals, National Academic Press 85: 23.
- Zimmerman M (1995) Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 16(2): 109-111.
- www.graphpad.com
- Ihcène B, Asma B, Amel B, Abdelfattah EF, Mahfoud M (2014) Antioxidant Effect of Alpha Lipoic Acid on Hepatotoxicity Induced by Aluminium Chloride in Rats. Int J Pharm Sci Rev Res 29(2): 19-25.
- Satyanarayana U, Chakrapani U (2010) Biochemistry, in Insulin, Glucose Homeostasis and Diabetes Mellitus. (3rd edn.), Arunabha Sen Books and Allied (P) Ltd., India. pp. 669-684.
- Valvi SR, Rathod VS (2011) Mineral Composition of Some Wild Edible Fruits from Kolhapur District. International Journal of Applied Biology and Pharmaceutical Technology 2(1): 392-396.
- Abhishek M, Thangadurai D, Shivanand B, Sangeetha J (2017) Proximate Analysis and Mineral Composition of Potential Minor Fruits of Western Ghats of India. Agronomy Series A. pp. 340-346.






























