Enzyme Catalysis Modulation Through Polysulfidation
Md. Morshedul Alam*
Department of Biochemistry and Microbiology, School of Health and Life Sciences, North South University, Dhaka, Bangladesh
Submission: July 12, 2024; Published: July 22, 2024
*Corresponding author: Md. Morshedul Alam, Department of Biochemistry and Microbiology, School of Health and Life Sciences, North South University, Dhaka, Bangladesh
How to cite this article: Md. Morshedul A. Enzyme Catalysis Modulation Through Polysulfidation Ann Rev Resear. 2024; 11(4): 555816. DOI: 10.19080/ARR.2024.11.555816
Abstract
Recent investigation in redox biology revealed the existence of a new biomolecule known as supersulfides, which are common in bacteria, mice, and human. Catenated sulfur atoms are defined as supersulfides. Some examples of supersulfides include cysteine per/tri-sulfide, glutathione per/tri-sulfides. Bunch of studies revealed various biological roles of the supersulfides such as signal transduction, protein modifications, inflammation, redox balance, understanding of host-pathogen interaction and so on. As research in this brand new filed is ongoing, a novel role of this biomolecule came in front and that was enzyme catalysis modulation capability of supersulfieds. In this review article, supersulfide-mediated enzyme catalysis modulation for a bifunctional enzyme, alcohol dehydrogenase 5 (ADH5) that has unique feature of GSNOR and FDH activity, is focused. For a long since, the mechanism of the bifunctional role of ADH5 was unknown. In this article, a focus was given on the supersulfidation-mediated regulation of ADH5 based on a recent finding of Kasamatsu et al (2023). This review will also unveil some possible clues to study some other enzymes/proteins functions due to polysulfidation-based posttranslational modification.
Keywords: Supersulfides; Polysulfur; Posttranslational modification; Redox biology; Enzyme
Abbreviations: ADH5: Alcohol Dehydrogenase 5; ROS: Reactive Oxygen Species; ETC: Electron Transport Chain; ETHE1: Ethylmalonic Encephalopathy Protein 1; GSSH: Glutathione Hydropersulfide; GSNOR: S-nitrosoglutathione Reductase; NADH: Nicotinamide Adenine Dinucleotide; FDH: Formaldehyde Dehydrogenase; PEG: Polyethylene Glycol
Introduction
Abundance of the presence of various types of macro or micro molecules in the biological systems, such as in human, plant or microorganisms, is a common scenario. Those molecules also play pivotal roles to modulate various biological reactions inside the body parts. Akin to other major or minor components of the earth or the living system, sulfur is considered as a major component of the nature in earth. It is the seventh most abundant molecule in higher vertebrate tissues. The total amount of sulfur in human body was quantified as 4400nmol [1]. It is also thought in the evolutionary biology that at the primitive age of earth, when there was no appearance of oxygen, sulfur was imagined to be utilized for respiration, which can be termed as “sulfur respiration”. During the evolution of life and when oxygen was abundant in nature, the lives on earth were adapted to use oxygen, which is chemically similar to sulfur, for respiration [2]. Afterwards, due to various biological needs or as byproducts, reactive oxygen species (ROS) and other free radicals such as nitric oxide also became the major substances in many biological processes or systems [3].
Recent study revealed the existence of a new bioactive molecule in living system, which is known as supersulfides [4]. Supersulfides are so defined since the sulfur species has catenated sulfur atoms that include hydropersulfides (RSSHs) and polysulfide species (RSSnR; R = hydrogen, and alkyl or cyclic sulfurs) [5] and are found in the cells or tissues at the submillimolar or millimolar level and exist in the cysteine residue side chains of proteins [6]. The unique feature of supersulfides is its dual redox reactivity to both nucleophiles and electrophiles, that paves the way of supersulfides to get engaged in diverse biochemical reactions [5,7,8].
Advancement of sulfur biology study unveils the mystery of various unknown biological conditions in the environment that may reflect the living system from the primitive stage of earth to the modern stage. One of the important characteristics of supersulfides is that they act as electrons acceptor produced by the mitochondrial electron transport chain (ETC) [5,7]. This means that in addition to cellular respiration, where oxygen acts as final electron acceptor in the ETC, sulfur respiration would be an additive where supersulfides act as electron acceptor and that could help us to understand the life during the primitive stage of earth when oxygen was not available but living creatures existed and possibly, they were alive due to sulfur respiration. Furthermore, the formation of supersulfide on protein having cysteine residues can dramatically alters the functional characteristics of many biologically active protein molecules, thereby influencing many cellular signaling pathways [5,9]. Thus, the enormously spreading research of sulfur biology is opening a new era of biotechnological research to promote the health and social wellbeing.
Supersulfide mediated enzyme activity modulation was reported for a mitochondrial enzyme, ethylmalonic encephalopathy protein 1 (ETHE1), a persulfide dioxygenase, which was reported to metabolize endogenous glutathione hydropersulfide (GSSH) to glutathione (GSH). This enzyme function is maintained by means of supersulfide catalysis of the Cys247 residue [10]. In this review article, other than many other biological significances, a novel catalytic role of polysulfur/polysufidation (i.e, supersulfides) process to modulate the enzymatic activity of a human bifunctional enzyme, alcohol dehydrogenase 5 (ADH5), has been focused.
Physiological roles of polysulfides or supersulfides
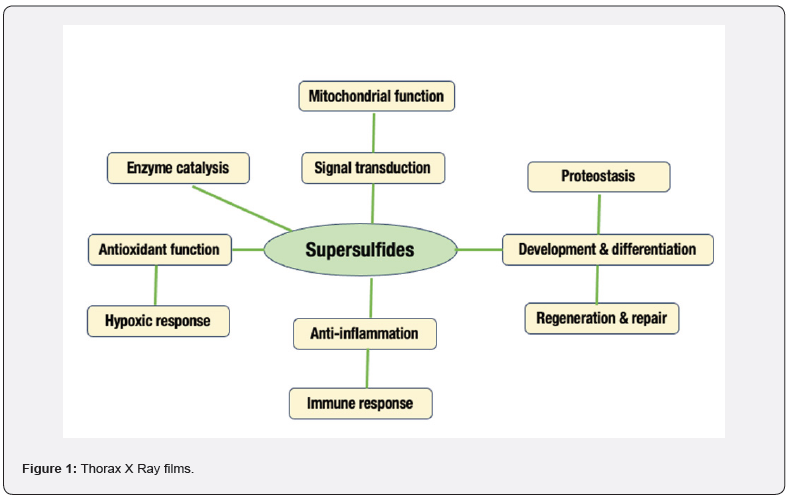
In a recent study, supersulfides are considered to be an important biomolecule in life science research [5]. They remain in the biological system in trace amount as low-molecular weight metabolites or cysteine side chain component in a protein. Some well-known examples of supersulfides include cysteine persulfide, glutathione persulfide, cysteine trisulfide, and glutathione trisulfide [6]. Due to their super-reactivity other than normal thiols to oxidative stress, they got prevalence to aging science research. This higher reactivity property of the supersulfides supported their various physiological roles that include anti-inflammatory functions [9,11], antioxidant functions [6,12], signal transduction [13], protein quality control [14], regulation of mitochondrial membrane potential [7], regulation of lipid peroxidation and ferroptosis [8] (Figure 1). Supersulfides also contribute to the understanding of disease pathogenesis related to nitric oxide signaling and also to take proper measure for various nitric oxide-related pathological situations such as cardiovascular, gastrointestinal, pulmonary, and neurodegenerative disorders [7].
Alcohol Dehydrogenase 5 (ADH5) Enzyme
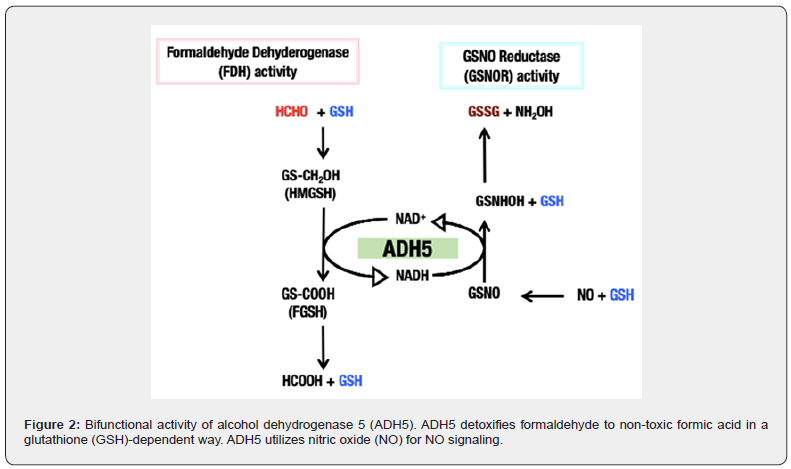
Alcohol dehydrogenase 5 (ADH5) is designated as a class III alcohol dehydrogenase and are highly conserved among prokaryotes to eukaryotes. It is also known as S-nitrosoglutathione reductase (GSNOR) having active center of zinc ion [15]. It is reported that ADH5 is a bifunctional enzyme (Figure 2) having both GSNOR activity, degradation of S-nitrosoglutathone (GSNO) to generate hydroxylamine (NH2OH) and glutathione (GSH/GSSG) using nicotinamide adenine dinucleotide (NADH), and GSH-dependent formaldehyde dehydrogenase (FDH) activity, oxidizes the substrate formaldehyde (H-CHO) to produce formate in the form of GSH conjugate using oxidized form of nicotinamide adenine dinucleotide (NAD+) [16,17]. So that ADH5 enzyme has both NO signaling pathway regulation and formaldehyde detoxification roles. Many studies demonstrated these functions of ADH5 in a separate study but not in a combined state to understand the key factor that is responsible for its dual nature.
Modulation of Enzyme Catalytic Function through Polysulfidation
Bunch of studies have been done by using Adh5-deficient mice to understand the pathological relevance of ADH5 such as NO signaling [18], hepatocellular carcinoma [19], steatohepatitis [20], cardiovascular functions [21] and so on. Though simple loss of function of ADH5 showed some relevance with pathogenesis of many diseases, but how the unique feature of bifunctional enzyme activities (i.e, GSNOR and FDH activity) of this enzyme is maintained was a big question to biologists. After getting the abundance of supersulfides in various tissues and mice or human plasma, a question was raised to know their biological relevance as well as their cooperation with biological macro or micro molecules. Akaike et al (2017) showed that a Cys174 residue located at zinc coordination site of ADH5 is heavily supersulfidated/polysulfidated in cells [5]. This observation actually gave a clue to the researchers that polysulfidation/supersulfidation of ADH5 would play a significant role to modulate its bifunctional activity.
Later on, Kasamatsu et al (2023) of the same group of Akaike, investigated that the highly supersulfidated Cys174 residue is critical to maintain the dual function of ADH5 by using both recombinant ADH5 protein in vitro and in vivo [22]. This is the very first report where supersulfidation regulates the switch on/off role of an enzyme, ADH5, and also to understand and develop a novel animal model to study the pathophysiology of cardiovascular diseases. In this study, it was observed that ADH5 wild type protein is highly supersulfidated at Cys174 residue, which was confirmed through polyethylene glycol (PEG)-conjugated maleimide (MAL)-labeling gel shift assay (PMSA), and the polysulfidation level was altered when Cys174 mutant ADH5 was investigated in the similar way. When enzymatic analysis was analyzed by using both wild type and mutant ADH5, which covered the GSNOR and FDH activity study, in that case a very novel finding was observed that showed that polysulfidation retains both GSNOR and FDH activity of ADH5, but when Cys174 mutant ADH5 was investigated, it showed that only GSNOR activity is gone but still retains FDH activity. Protein structural alteration effect due to this mutation was not observed at all. This means that supersulfidation of ADH5 at Cys174 residue is critical to switch on/off the GSNOR activity of ADH5, which actually correlates the dual enzyme function of this protein/enzyme. Thus, a novel property of supersulfides is that it has not only protein side chain modification capacity, i.e, post-translational modifications, for signal transduction but also to modulate bifunctional enzyme activity.
Conclusion
Persulfides and polysulfides are formed from the catenation of sulfur atoms, which add a unique chemical feature and thus act as supersulfides. As supersulfides have bipolar activities, so that they harbor the potential to chemically or enzymatically modify any biological reactions, for what still scientists don’t understand the real molecular mechanism such as autophosphorylation of some important biomolecule. The various pathological and physiological roles of supersulfides may be considered as the tip of the iceberg of supersulfide biology. Additional studies are now warranted to unveil the vast knowledge in this brand new supersulfide biology field.
Acknowledgement
I acknowledge, as a former member, Motohashi and Akaike’s group for their endless hard work in this new research area and also other scientists who are working in this field to disclose many unknown scientific findings related to supersulfide biology.
References
- Ingenbleek Yves (2006) The nutritional relationship linking sulfur to nitrogen in living organisms. J Nutrition 136(6): 1641S-1651S.
- Ogata S, Matsunaga T, Jung M, Barayeu U, Morita M, et al. (2023) Persulfide biosynthesis conserved evolutionarily in all organisms. Antioxid Redox Signal 39(13-15): 983-999.
- Ernst L, Steinfeld B, Barayeu U, Klintzsch T, Kurth M, et al. (2022) Methane formation driven by reactive oxygen species across all living organisms. Nature 603: 482-487.
- Zhang T, Akaike T, Sawa T (2023) Redox Regulation of Xenobiotics by Reactive Sulfur and Supersulfide Species. Antioxid Redox Signal 40(10-12): 679-690.
- Akaike T, Ida T, Wei FY, Nishida M, Kumagai Y, et al. (2017) Cysteinyl- tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nature Communications 8(1): 1177.
- Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, et al. (2014) Reactive cysteine persulfides and S-polythiolation regulate oxi- dative stress and redox signaling. Proc Natl Acad Sci USA 111(21): 7606-7611.
- Alam MM, Kishino A, Sung E, Sekine H, Abe T, et al. (2023) Contribution of NRF2 to sulfur metabolism and mitochondrial activity. Redox Biol 60: 102624.
- Barayeu U, Sawa T, Nishida M, Wei FY, Motohashi H, et al. (2023) Supersulfide biology and translational medicine for disease control. Br J Pharmacol p. 1-16.
- Matsunaga T, Sano H, Takita K, Morita M, Yamanaka S, et al. (2023) Supersulphides provide airway protection in viral and chronic lung diseases. Nature Commun 14: 4476.
- Jung M, Kasamatsu S, Matsunaga T, Akashi S, Ono K, et al. (2016) Protein polysulfidation-dependent persulfide dioxygenase activity of ethylmalonic encephalopathy protein 1. Biochem Biophys Res Commun 480(2): 180-186.
- Takeda H, Murakami S, Liu Z, Sawa T, Takahashi M, Izumi Y, et al. (2023) Sulfur metabolic response in macrophage limits excessive inflammatory response by creating a negative feedback loop. Redox Biol 65: 102834.
- Millikin R, Bianco CL, White C, Saund SS, Henriquez S, et al. (2016) The chemical biology of protein hydropersulfides: Studies of a possible protective function of biological hydropersulfide generation. Free Radic Biol Med 97: 136-147.
- Nishimura A, Shimoda K, Tanaka T, Toyama T, Nishiyama K, et al. (2019) Depolysulfidation of Drp1 induced by low-dose methylmercury exposure increases cardiac vulnerability to hemodynamic overload. Sci Signal 12(587): eaaw1920.
- Dóka É, Ida T, Dagnell M, Abiko Y, Luong NC, et al. (2020) Control of protein function through oxidation and reduction of persulfidated states. Sci Adv 6(1): eaax8358.
- Liu L, Hausladen A, Zeng M, Que L, Heitman J, et al. (2001) A metabolic enzyme for S- nitrosothiol conserved from bacteria to humans. Nature 410(6827): 490-494.
- Koivusalo M, Baumann M, Uotila L (1989) Evidence for the identity of glutathione-dependent formaldehyde dehydrogenase and class III alcohol dehydrogenase. FEBS Lett 257: 105-109.
- Koivusalo M, Lapatto R, Uotila L (1995) Purification and characterization of S-formylglutathione hydrolase from human, rat and fish tissues. Adv Exp Med Biol 372: 427-433.
- Wei W, Yang Z, Tang CH, Liu L (2011) Targeted deletion of GSNOR in hepatocytes of mice causes nitrosative inactivation of O6-alkylguanine-DNA alkyltransferase and increased sensitivity to genotoxic diethylnitrosamine. Carcinogenesis 32: 973-977.
- Tang CH, Wei W, Hanes MA, Liu L (2013) Hepatocarcinogenesis driven by GSNOR deficiency is prevented by iNOS inhibition. Cancer Res 73(9): 2897-2904.
- Goto M, Kitamura H, Alam MM, Ota N, Haseba T, et al. (2015) Alcohol dehydrogenase 3 contributes to the protection of liver from nonalcoholic steatohepatitis. Genes Cells 20(6): 464-480.
- Lima B, Forrester MT, Hess DT, Stamler JS (2010) S-Nitrosylation in cardiovascular signaling. Circ Res 106(4): 633-646.
- Kasamatsu S, Nishimura A, Alam MM, Morita M, Shimoda K, et al. (2023) Supersulfide catalysis for nitric oxide and aldehyde metabolism. Sci Adv 9(33): eadg8631.






























