- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Biomarkers in Supporting Personalized & Precision Medicine Initiatives and their Global Impact in the Upgraded Healthcare-Related Clinical Practice
Sergey Suchkov1-6,9*, Daniel Scherman11, Alan Wu15,16, Lidya Kadyrova13, Shawn Murphy7,8, Vladimir Zemskov14, David Smith12, Hiroyuki Abe9,10, Valentina Demidova14, David Smith11
1Russian University of Medicine, Moscow, Russia
2The Russian Academy of Natural Sciences, Moscow, Russia
3EPMA, Brussels, EU
4PMC, Washington, DC, USA
5AMEE, Dundee, Scotland
6New York Academy of Sciences, USA
7MGH, Boston, MA, USA
8Harvard Medical School, Boston, MA, USA
9ISPM, Tokyo, Japan
10Abe Cancer Clinic, Tokyo, Japan
11Centre de Recherche Pharmaceutique de Paris (CRP2); Faculté de Pharmacie, Université Paris Descarte, Paris, France
12Mayo Clinic, Rochester, MN, USA
13Department of Neurophysiology of Kazan State Medical Academy, Kazan, Russia
14National Medical Research Center for Surgery named after A.V. Vishnevsky, Moscow, Russia
15Lab of Clinical Pharmacogenomics, San Francisco General Hospital, San Francisco, CA, USA
16Dept for Pharmacogenomics, UCSF Medical School, San Francisco, CA, USA
Submission: March 12, 2024; Published: April 04, 2024
*Corresponding author: Sergey Suchkov, Sergey Suchkov, Russian University of Medicine, Moscow, Russia, New York Academy of Sciences, USA & ISPM, Tokyo, Japan
How to cite this article: Sergey Suchkov*, Daniel Scherman, Alan Wu, Lidya Kadyrova, Shawn Murphy, et al. Biomarkers in Supporting Personalized & Precision Medicine Initiatives and their Global Impact in the Upgraded Healthcare-Related Clinical Practice. Ann Rev Resear. 2024; 11(1): 555801. DOI: 10.19080/ARR.2024.11.555801
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Abstract
Personalized and precision medicine (PPM) relies on specific biomarkers to guide clinical decisions, distinguishing between predictive and prognostic biomarkers. Biomarkers are crucial in diagnosis, risk assessment, treatment guidance, and monitoring, with the potential to improve treatment outcomes. Biomarkers are clinically significant to a wide range of medical conditions, including cardiovascular diseases, autoimmune disorders, and neurodegenerative diseases. In cancer research, biomarkers enable risk assessment, diagnosis, prognosis determination, treatment prediction, therapy monitoring, and revolutionizing clinical decision-making. Designing trials for biomarker-guided therapy presents numerous challenges. Despite these, biomarker-guided trials are crucial for advancing personalized medicine and improving patient outcomes. New genera-tion of biomarkers, particularly microRNAs are gathering attention into the realm of personalized and precision medicine due to their diagnostic, prognostic, and predictive biomarker potential. However, new types of biomarkers need to be researched and implemented to harness this poten-tial. Additionally, this article discusses the integration of biomarkers into clinical practice, the direction of potential research, and the transformative impact new types of biomarkers can have on personalized medicine. Ultimately, the article emphasizes the significance of biomarker re-search in advancing personalized healthcare interventions, improving patient outcomes, and re-shaping the healthcare landscape.
Keywords: Biomarker, Biomarker classification, Clinical trial, Companion diagnostics (CDx), Drug development, Personalized and Precision Medicine, Personalized treatment, Bi-odesign, Predictive biomarker, Prognostic biomarker
Abbreviations: PPM: Personalized and precision medicine; CFTR: Cystic fibrosis trans-membrane conductance regulator; HLA: Human leukocyte antigen allele; HIV: Human immuno-deficiency virus); PSA: Prostate-specific antigen; COPD: Chronic obstructive pulmonary disor-der; CRP: C-reactive protein; EGFR: Epidermal growth factor receptor; PPO: Personalized and precision oncology; miRNAs: microRNAs; CTCs: Circulating Tumor; CPAN: Cancer protein as-sociation network; PPI: Protein-protein interactions; NBBs: Network-based biomarkers; Abs: Antibodies; CatAbs: Catalytic activity antibodies; MS: Multiple sclerosis
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Introduction
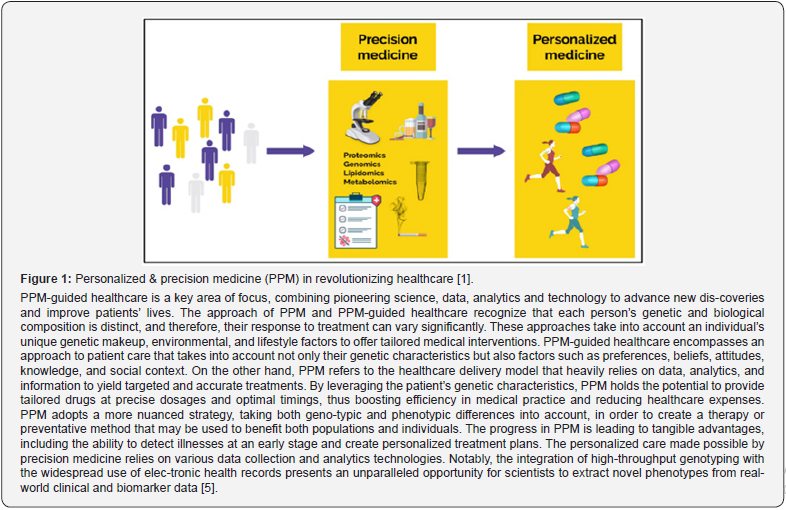
The area of personalized and precision medicine (PPM) as an upgraded model of healthcare services is an area of daily clinical practice, that involves the use of measuring specific and/or targeted biomarkers as an area of clinical interest. The success in the management of dis-orders directly depends on the stage of the pathological process, and a wide range of biomarkers, due to their high specificity, can diagnose a disease and/or predict the risks of its development at the preclinical stage with almost 100% probability. Meanwhile, marker molecules are used to de-termine, in particular, the state of cell damage processes, a number of functionally important bi-omolecules, as well as the presence of metabolites or precursor proteins, which are detected thanks to innovative OMICS-technologies such as genomics, proteomics, transcriptomics and metabolomics (Figure 1).
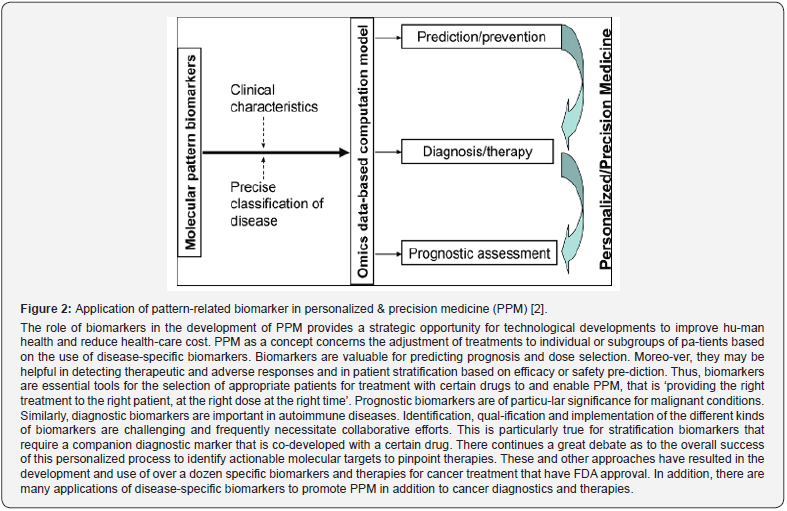
Biomarkers play a crucial role in understanding and monitoring the intricacies of the hu-man body. They offer valuable insights into biological processes, disease diagnosis, treatment effectiveness, and patient outcomes, enabling researchers and clinicians to make informed clinical decisions. The identification of biomarkers to support decision-making is central to PPM, in clinical scenarios. The difference between a biomarker’s prognostic and predictive value can be high-ly useful in charting a treatment plan for patients and determining treatment outcomes (Figure 2). PPM relies on validated biomarkers with which to better classify patients by their proba-ble disease risk, prognosis and/ or response to treatment. The challenge of PPM can be seen in two halves: identifying predictive biomarkers and prognostic markers.
Identifying predictive biomarkers, which guide the development/use of tailored therapies - among common predictive biomarkers, such as in cystic fibrosis transmembrane conductance regulator (CFTR) mutations that can identify patients who respond more favorably than others to particular treatments; human leukocyte antigen allele (HLA)–B*5701 genotype to evaluate HIV patients before the onset of abacavir treatment to identify patients at risk for severe skin reac-tions; [3]; breast cancer genes- BRCA 1 and 2 mutations as a predictive biomarker to identify patients (women with platinum-sensitive breast cancer), who are likely to respond to ADP-ribose-PARP inhibitors [4]. Identifying prognostic markers, which guide other aspects of care and clini-cal trial planning, i.e. prognostic markers can be considered as covariates for stratification – breast cancer genes, BRCA 1 and 2, as prognostic biomarkers that can help determine the likeli-hood of recurrence of breast cancer; prostate-specific antigen (PSA) as a prognostic biomarker for assessing disease progression in prostate cancer patients; plasma fibrinogen can be used as a prognostic biomarker for patients with the chronic obstructive pulmonary disorder (COPD) to determine risk for exacerbation; C-reactive protein (CRP) as a prognostic biomarker that can be used for patients with a history of myocardial infarction or unstable angina to identify risk of re-current coronary artery disease [5]. The rapid development of computation biology, systems biology, and multi-OMICS is driving the development of pattern recognition to discover reliable molecular pattern biomarkers for biomarker-driven targeted treatment [6].
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Biomarker categories in clinical practice
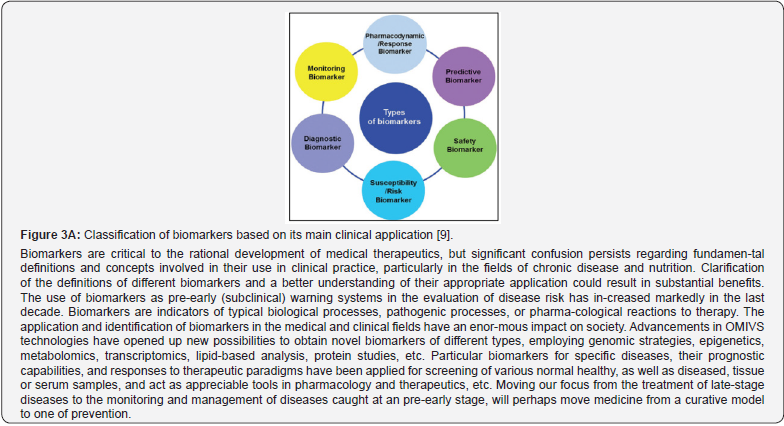
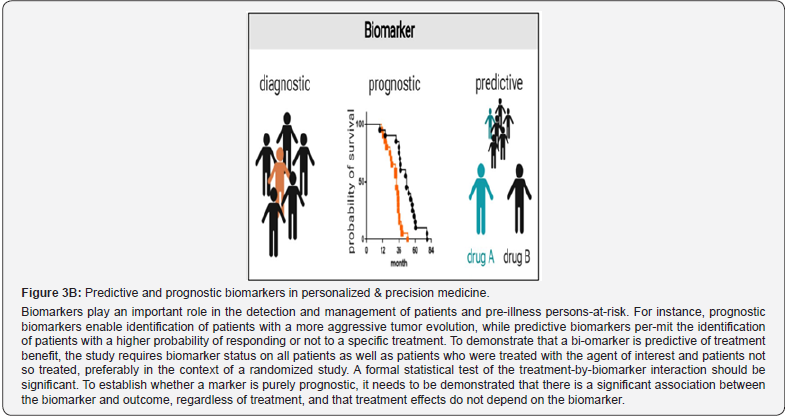
To establish whether a marker is purely prognostic, it needs to be demonstrated that there is a strong association between biomarker-related profile and the outcome, regardless of treatment. The latter can be used in diagnostics, as well as in assessment of disease severity, risk stratification, prediction, guide clinical decisions, and help pick appropriate treatment, modify therapy, and response to it. In this review, we will analyze what characteristics a biomarker should have and how to ensure its usefulness, and we will review the biomarkers that in our opinion can make their knowledge more useful to the reader in their clinical practice, with a future perspective. Finally, a biomarker may have both predictive and prognostic implications [7]. In a broader strategic sense demonstrating the evidence-based clinical value, there are key categories of biomarkers (Figure 3A, 3B), including:
i. Diagnostic biomarkers: to identify individuals with a disease or condition of interest or to define a subset of the disease ii. Prognostic biomarkers: indicate the likelihood of a clinical event, disease recurrence, or progression.
iii. Predictive biomarkers: to identify individuals who are likely to experience a favorable or unfavorable effect from a specific intervention or exposure
iv. Safety biomarkers
v. Pharmacodynamic (response) biomarkers
vi. Monitoring biomarker
vii. Susceptibility (risk) biomarkers [8].
Analyzing and assessing the above-mentioned diagram, stress that predictive and phar-macodynamic biomarkers would play a crucial role in identifying patients or persons at risk who are more likely to respond favorably to specific treatments [10]. By unraveling the underlying molecular mechanisms associated with treatment response, these biomarkers pave the way for targeted interventions, optimizing treatment outcomes and minimizing unnecessary adverse ef-fects. For instance, the identification of EGFR-related mutations in lung cancer, determines the response to EGFR inhibitors, leading to improved treatment efficacy and patient survival rates. The advent of those biomarkers has revolutionized the field of PPM, where treatments are tai-lored to individual patients based on their unique disease characteristics. By providing insights into the likelihood of treatment response, predictive biomarkers empower clinicians to make in-formed decisions and optimize therapeutic interventions [11].
In contrast to the fully validated and FDA-approved biomarkers, many exploratory bi-omarkers and biomarker candidates have potential applications. Prognostic biomarkers are of par-ticular significance for malignant conditions and monitoring cancer related conditions. Similarly, canonical diagnostic biomarkers are important in autoimmune diseases. Disease severity bi-omarkers are helpful tools in the treatment for chronic inflammatory diseases. Identification, qualification, and implementation of the different kinds of biomarkers are challenging and fre-quently necessitate collaborative efforts. This is particularly true for stratification biomarkers that require a companion diagnostic marker (theranostic) that is co-developed with a certain drug [12]. Theranostics is considered a fusion of diagnosis and medication. It helps to optimize the rationalization of safety, effective results and overall drug development. The latter in the future of PPM, being and serving as a valuable guidance, would play a crucial role in clinical practice since possessing their accuracy is crucial for the success of the therapeutic, preventive, prophylac-tic, and rehabilitative choice (Figure 4A,B).
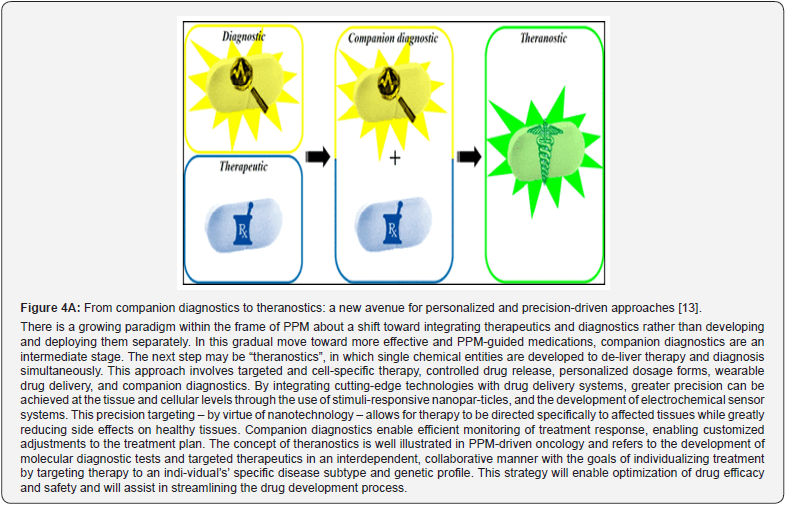
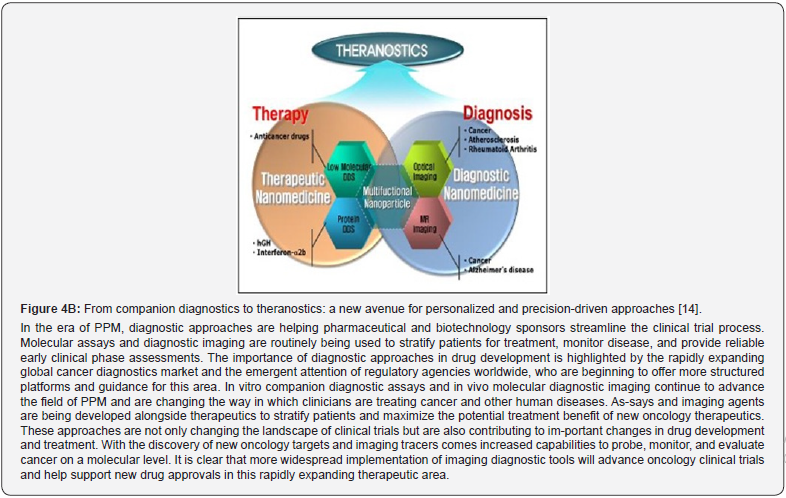
Efforts to discover next-generation biomarkers for clinical use have been significant, yet their implementation remains low [15-17]. PPM leverages advanced biomarker-driven technologies to inform evidence-based decisions across disease diagnosis, treatment, prediction, preven-tion, and prophylaxis. Utilizing molecular stratification, PPM enhances medication selection, re-duces adverse effects, and shifts focus from reaction to prevention [18,12]. Biomarkers derived from genes, proteins, and interactomes play a pivotal role in predicting individual responses to therapies and guiding personalized treatment strategies.
Questions persist regarding best practices for extracting prioritized biomarkers in PPM-driven research, amidst the era of OMICS-technologies and Big Data. Biomarkers are integral to the development of PPM-driven technologies, aiding in clinical decision-making, medical prod-uct development, and drug discovery. They serve as indicators of normal and pathogenic processes, contributing to drug target selection, patient stratification, and safety assessment in drug development [15,19].
The molecular heterogeneity of living systems offers a rich source of candidate bi-omarkers, necessitating controlled error rates in statistical models. Biomarkers can signify various health or disease characteristics, acting as indicators of disease trait, state, or progression. Rele-vant biomarkers define patient subgroups, informing evidence-based medical decisions [20- 22]. Expectations for precision tools like PSA and specific gene mutations are heightened by the po-tential of Big Data to translate into clinically relevant information [23].
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Biomarkers and Personalized and Precision Oncology (PPO)
Biomarkers are extremely important in cancer research and Personalized and Precision Oncology (PPO). They are crucial for risk assessment, screening, differential diagnosis, prognosis determination, prediction of disease recurrence and response to therapy, and progression moni-toring [11,24,25]. With cuttingedge proteomic and genomic technologies, DNA and tissue microarrays, gel electrophoresis, mass spectrometry, and protein assays, as well as improved bioin-formatics tools, the evolution of biomarkers to reliably assess the results of cancer mitigation and therapy is now possible. Looking forward, a urine or a serum test for each stage of cancer may drive clinical decision-making, complementing, or even replacing presently available invasive methods [15,26].
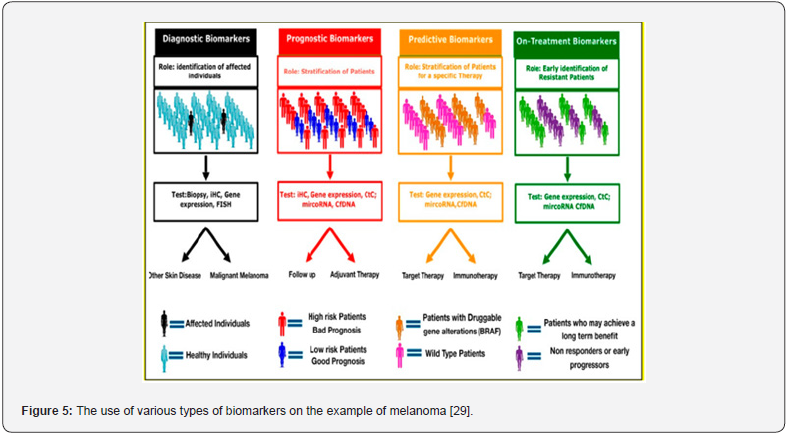
Predictive biomarkers help to optimize therapy decisions, as they provide information on the likelihood of response to a given chemotherapeutic. Among the prognostic factors that identify patients with different outcome risks (e.g., recurrence of the disease), the following factors can be distinguished: somatic and germline mutations, changes in DNA methylation that lead to the enhancement or suppression of gene expression, the occurrence of elevated levels of mi-croRNA (miRNA) capable of binding specific messenger RNA (mRNA) molecules, which af-fects gene expression, as well as the presence of circulating tumor cells (CTCs) in blood, which leads to a poor prognosis for the patient. Biomarkers for personalized oncology are used mainly in molecular diagnostics of chronic myeloid leukemia, colon, breast and lung cancer, and recently in melanoma. They are successfully used in the evaluation of the benefits that can be achieved through targeted therapy or in the evaluation of toxic effects of the chemotherapeutic used in the therapy [27,28] (Figure 5).
Due to the individualization of cancer therapy, the identification of cancer-and oncology-specific biomarkers has become a foremost goal for cancer researchers [2,15]. The common usage of PSA in prostate cancer screening has prompted investigators to look for appropriate bi-omarkers for screening other kinds of cancer. Targeted medicines, such as Iressa® (gefitinib), Gleevec® (imatinib), and Herceptin® (trastuzumab), are currently available and may benefit from a more targeted treatment based on diagnostic testing. Clinically, cancer-related families of biomarkers may help identify individuals who are most likely to react to a medication, enable real-time monitoring of treatment effectiveness, or detect early indications of drug toxicity. Furthermore, biomarkers are heavily used in go/no-go decision-making throughout the drug development cycle, from early discovery to preclinical as-sessment. Meanwhile, with the emergence of more sensitive and specific technologies that are now able to be run in clinical settings and the ability to accurately measure biomarkers, there is a need to understand how biomarkers are defined, and how they are used in conjunction with drug treatment or with the frame of protocols of clinical trials [30].
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Biomarkers of the next step generation
Meanwhile, a principally new generation of biomarkers is required that define all aspects of the variability of unified system indicators. For instance, miRNAs circulating are attracting interest in the burgeoning field of PPM and associated subfields, with data supporting their di-agnostic, prognostic, and predictive biomarker potential. MicroRNAs (miRNAs) are small non-coding RNA molecules, which modulate genetic expression and which in recent years have emerged to have enormous potential as biomarkers. A schematic diagram showing miRNA bio-genesis, modes of their secretion into body fluids, RNA extraction and quantitative approaches. In future, clinical decisions may be made based on the expression levels of miRNAs (Figure 6) [22].
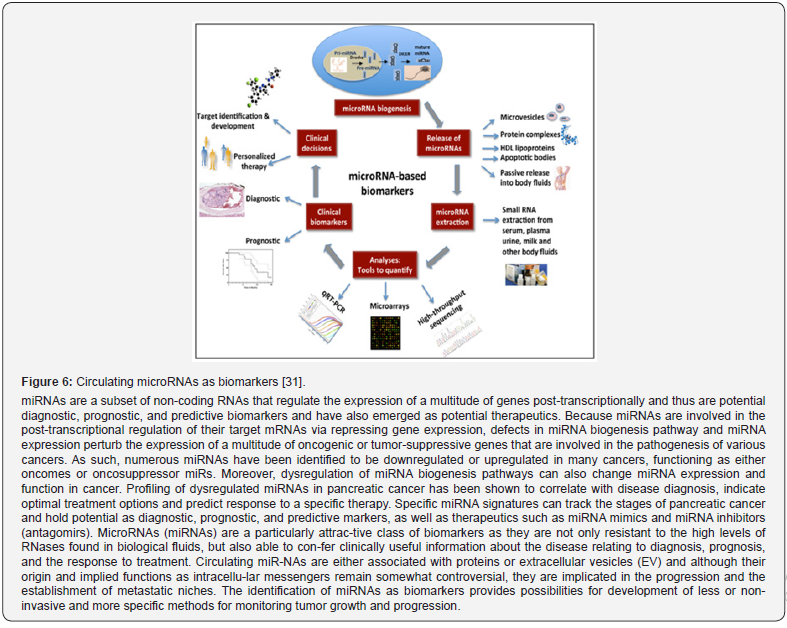
The tight association of miRNAs with several cancer-related processes makes them un-doubtedly connected to the effect of specific cancer drugs inducing either resistance or sensitiza-tion. In this context, personalized medicine through miRNAs arose recently with the discovery of single nucleotide polymorphisms in the target binding sites, in the sequence of the miRNA itself, or miRNA biogenesis-related genes, increasing risk, susceptibility, and progression of mul-tiple types of cancer in different sets of the population. miRNA markers show that they can ena-ble a wide range of diseases to be diagnosed before clinical symptoms are manifested, and they can help to assess a patient’s response to therapy to correct and personalize treatments.
The depicted scenario implies that the overall variation displayed by these small non-coding RNAs has an impact on patient-specific pharmacokinetics and pharmacodynamics of cancer drugs, pushing on a rising need for personalized treatment. Indeed, miRNAs from either tis-sues or liquid biopsies are also extensively studied as valuable disease biomarkers for early recog-nition, progression, and prognosis. Despite the lack of standardized protocols regarding the use of miRNAs in current clinical practice, they constitute a reliable tool for future use. These mole-cules meet most of the required criteria for being an ideal biomarker, such as accessibility, high specificity, and sensitivity. Effective miRNA profiling calls for reproducible, sensitive, and specific tools with turn-around times fast enough to support biodesign-inspired translational research and applications into what can be a rapidly changing disease progression and treatment environment. Circulating miRNAs became one of the most promising biomarkers in oncology for early diagnosis, progno-sis, and therapeutic response prediction [32,33].
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Circulating tumor cells (CTCs) in clinical practice
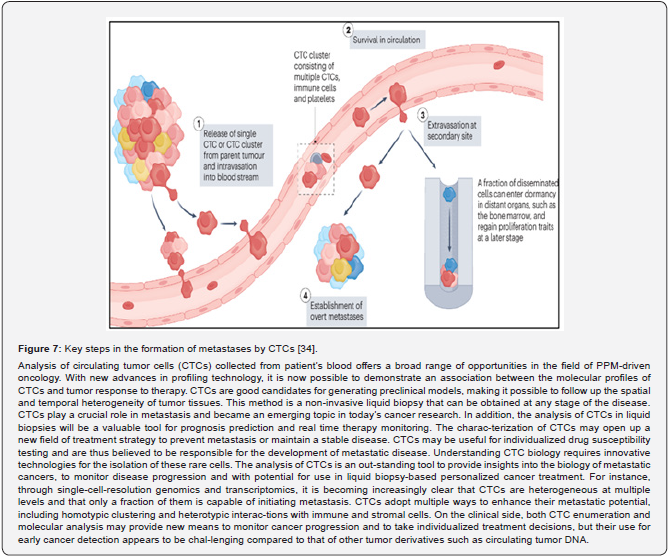
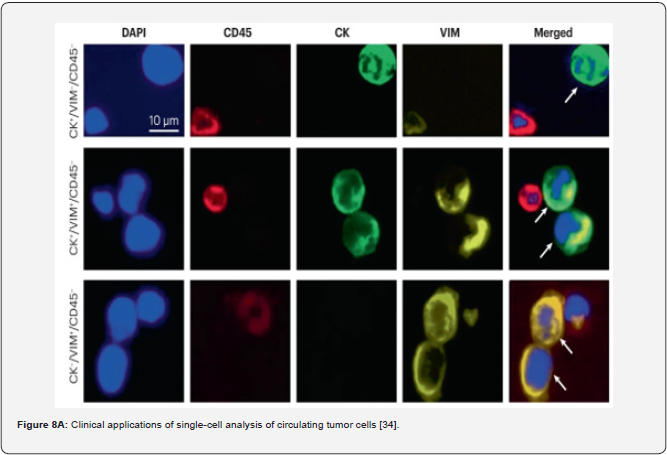
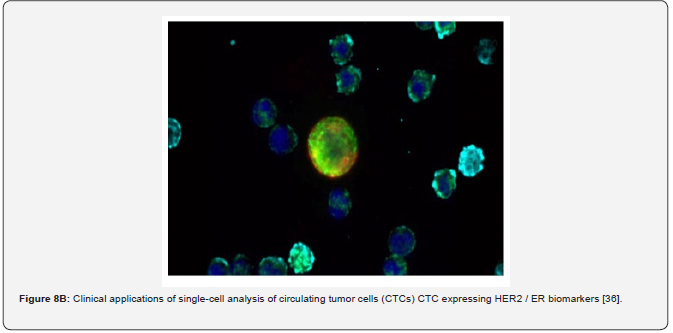
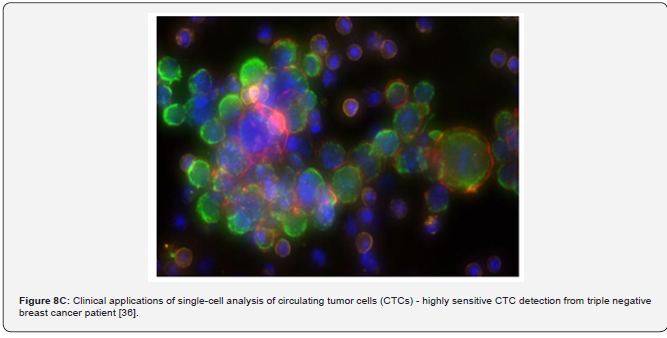
The other example of the next step generation biomarkers is circulating tumor cells (CTCs), illustrating subclinical stages and predictive risks as applicable to tumor progression [34]. CTCs as a biomarker of the latest generation would make up a minute fraction of the total num-ber of cells circulating in blood. CTCs can be released from the primary tumour into the blood-stream and may colonize distant organs giving rise to metastasis (Figure 7) [32,35]. CTCs as a kind of cellular biomarker that is detected in patients with early stage cancers and, owing to their association with metastasis, might indicate the presence of aggressive disease, thus providing a possible means to expedite diagnosis and treatment initiation for such patients while avoiding overdiagnosis and overtreatment of those with slow-growing, indolent tumors. It is estimated that more than 90% of cancer mortality is caused by distant metastasis. Investigating CTCs provides important tumor information since these cells are already related to metastatic disease; broken free from the tumor mass, transited through interstitial tissues, and intravasated into the vasculature. Understanding and counteracting this process is essential to managing can-cer. The utility of CTCs as an early diagnostic tool has been investigated and confirmed (Figure 8A-C).
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
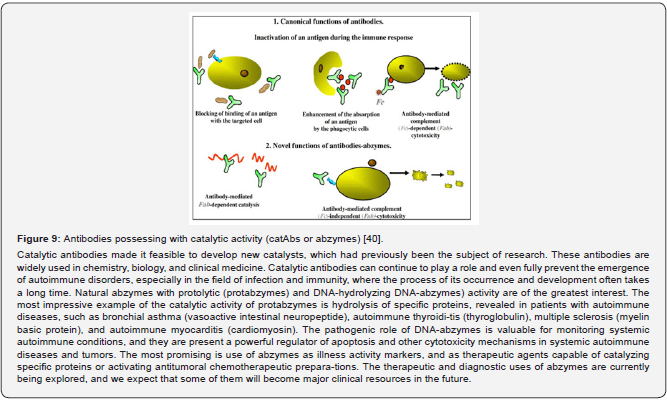
Сatalytic antibodies or abzymes
Novel biomarkers may also identify specific pathways involved in risk, where drugs inter-rupting such mediator biotargets have not yet been explored in appropriately designed trials [30,37,26]. Regarding biomarkers of the latest innovative trends, let me add that along with ca-nonical antibodies (Abs) serving a crucial role as biomarkers in clinical settings, some of the Ab-based families are Abs possessing with catalytic activity (catAbs or abzymes) and thus belong to Abs with a feature of functionality [38]. The immune system provides a highly evolved natural process to generate one class of complex biomarkers-the antibodies. A combination of the two could be exploited to generate new classes of molecules with novel functions. A catalytic antibody is a sort of natural artificial enzyme - it is a natural protein synthesized by the usual biological processes and is intended to catalyze a reaction for which no real enzyme is available [39].
CatAbs (or abzymes) are multivalent Igs, presumably of IgG isotype, endowed with a ca-pacity to hydrolyze Ags. The enzymatic activity is located in the Fab fragment of the Ig molecule, which endows such antibodies with the ability to bin specific antigens and hydrolyze them. Proteolytic Abs (or Abproteases) represent a significant portion of the family of abzymes that PPM uses to target specific Ags (Figure 9). Because of their Ag specificity, Ab-proteases also may be used as biomarkers able to con-trol autoimmune disease progression to transform from subclinical into clinical stages, and to predict complications. Moreover, sequence-specific Ab-proteases have proved to be greatly in-formative and thus valuable as biomarkers to monitor autoimmune diseases at both subclinical and clinical stages while demonstrating their predictive value for the development of the disorder [40-42].
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Network-based biomarkers (NBBs)
Moreover, following the clinical aims and objectives of the next step generation and hav-ing a complete understanding of a drug’s pathway, interactome, and network interactions could expedite the identification of sensitizing mutations, drug interactions, or the risks of drug combi-nations to guide biomarker discovery, including simple, combinatorial, and network-based bi-omarkers (NBBs). Today the experts may also identify specific pathways and interactome-based networks involved in diseases for which drugs have not yet been explored in appropriately de-signed trials, and being set up for: normal (physiological) and pathological conditions (Figure 10 A,B).
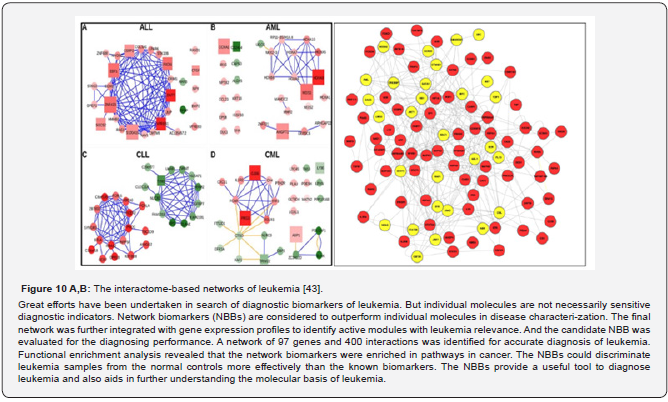
Those networks may serve as sources of network-based biomarkers (NBBs) to secure di-agnostic, predictive, prognostic and monitoring-related aims and objectives of the next step gen-eration. In this sense, NBBs help determine the probability of developing chronic pathologies, autoimmunity, or cancerpredisposed conditions. Key factors contributing to the growth of the global NBBs-related healthcare services market include high prevalence of chronic autoimmune diseases and cancer; rising adoption of biomarkers for diagnostic, predictive, and prognostic ap-plications, as well as increasing application in drug discovery and development. A NBB using constructed protein association networks is a useful tool to highlight the pathways and mechanisms of the lung carcinogenic process and, more importantly, provides po-tential therapeutic targets to combat cancer. From a systems perspective, the constructed net-work-based biomarker further evaluated the targeted carcinogenic process by the use of significant protein identification and diagnostic evaluation. More importantly, the significant proteins identified by the NBBs give mechanistic insights into the carcinogenic process and provide po-tential therapeutic targets to combat cancer in daily clinical practice of medicine [44].
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Biomarker-guided clinical trial design
Innovative clinical trial designs are needed to address the difficulties and issues in the development and validation of biomarker-based personalized therapies. Designing trials of biomarker- guided therapy has many challenges, including:
i. Being almost always unblinded, they are prone to bias
ii. The control group, most frequently the ‘usual care’ group,
is opened to contamination and has inevitably better outcomes than in real non-trial ‘usual care’
iii. Being per essence ‘strategy-trials’ rather than simple intervention trials, causality is diffi-cult to establish
iv. Therapy optimization as a result of a change in the tested biomarker may be left to the de-cision of the investigator, only instructed to follow best guideline medical therapy, or de-cided per protocol using more or less sophisticated algorithms, which, although guideline-based, may vary according to the protocol [30,45].
Validated biomarkers are essential for improving diagnoses, molecular targeted therapy, and therapeutic benefits across various diseases. Despite recent progress, the path to clinically validated biomarkers remains challenging, often creating a gap between research and application. Clinical trials are necessary to confirm the properties of emerging treatments and associated biomarkers, influencing daily clinical practices and regulatory reporting before professional or commercial release. Biomarkers also play a role in facilitating drug repurposing and guiding patient care decisions within clinical settings and drug development processes. The involvement of biomarkers in clinical practices will be more and more com-mon in the next 5–10 years because of the development in medical-related biological and trans-disciplinary research, as well as in biodesign-inspired and biotech-driven translational applica-tions. More clinical questions need to be answered about the biomarker and its role in the disease process and therefore more biomarker-related clinical trials will be designed to answer those spe-cific questions. More flexible trials serving multiple purposes are expected due to the intricate relationship between biomarkers and the disease.
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Future applications of biomarkers in healthcare
Biomarkers are indicators of typical biological processes, pathogenic processes, or phar-macological reactions to therapy. And you might see from the above-mentioned, that biomarkers can be used, along with tools in clinical practice, as drug development tools and can be incorpo-rated into drug development through the drug approval process, scientific community consensus followed by regulatory acceptance, and biomarker qualification. This would offer a new way to optimize treatment, decrease rehabilitation costs, and facilitate building new products and ser-vices in this area.
Numerous compounds have been tested as potential biomarkers for multiple possible ap-plications within PPMguided care but none is or will ever be sufficiently specific or sensitive for the heterogeneous syndromes of critical illness. New technology and access to huge patient data-bases are providing new biomarker options and the focus is shifting to combinations of several or multiple biomarkers rather than the single markers that research has concentrated on in the past. Biomarkers will increasingly be used as part of routine clinical practice in the future, comple-menting clinical examination and physician expertise to provide accurate disease diagnosis, pre-diction of complications, personalized treatment guidance, and prognosis. For instance, biomarkers, defined as alterations in the constituents of tissues or body flu-ids, provide a powerful approach to understanding the spectrum of cardiovascular diseases and chronic autoimmune myocarditis with applications involving screening, diagnosis, prognostica-tion, prediction of disease recurrence, and therapeutic monitoring. The unique diagnostic poten-tial of specific biomarkers and their efficacy correlates with phenotypical expression, covering neuroinflammation and neurodegeneration, including the applications of biomarker-based strate-gy in multiple sclerosis (MS), Parkinson and Alzheimer diseases.
A comprehensive understanding of the relevance of each cancer biomarker will be very important not only for diagnosing the disease reliably, but also help in the choice of multiple therapeutic alternatives currently available that are likely to benefit the patients. Cancer bi-omarkers are broadly categorized into three divisions based on the specific association with diagnostic, diagnostic, predictive and prognostic biomarkers. The therapeutic potential of different biomarkers and their use in clinical trials has also been discussed. Since the use of biomarkers as the pre-early (subclinical or pre-illness) warning systems in the evaluation of disease risk has increased markedly in the last decade. And thus the application and identification of biomarkers in the medical and clinical fields have an enormous impact on society. Despite the recent advancements, a comprehensive approach in biomarker biogenesis is required to integrate the available information and to translate them as tools of prognostic and diagnostic potential.
Biomarkers of the future would be used for:
i. Screening the general population or individuals at risk (panels of screening and predisposi-tion biomarkers)
ii. The detection of the presence of a particular type of cancer (panels of diagnostic and prognostic biomarkers).
iii. Monitoring the progression of autoimmune inflammation and predicting the complica-tions and outcome (panels of prognostic biomarkers).
iv. Understanding whether a patient will benefit from a specific drug treatment (panels of predictive biomarkers)
v. Evaluating the drug’s efficacy and optimizing the treatment, providing the tool to tailor treatment for individual autoimmunity-related patients or persons at risk (panels of pharmacodynamics biomarkers)
Meanwhile, a number of limitations of multimarker-based panels should be acknowl-edged. These include potential multiplexing and analytical challenges in assaying multiple biomarkers at once as well as the challenges of interpretation for the clinician due to different cut-offs for each of the separate markers [20]. Nevertheless, it can be anticipated that scoring calcula-tors and algorithms will increasingly use circulating biomarkers in combination with clinical vari-ables to allow appropriate surveillance and fully informed counselling of the patients, persons-at-risk, their families and other stakeholders in the process of patient care.
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
Conclusion
Biomarkers have gained significant scientific and clinical importance in the realm of PPM and related fields, offering potential benefits throughout the disease process. The oncology seg-ment is the largest area for biomarker growth. Biomarkers can be used for early detection, esti-mate prognosis, guide selection of targeted therapies (companion diagnostics), and monitor response to treatment. The increased demand for rapid and accurate diagnostic OMICS-tools, an increase in the global cancer burden, and an unmet need for more specific, personalized, thera-peutic targets for cancer patients and pre-cancer persons-at-risk will continue to drive growth in the market, which is really growing.
The current value of the global biomarkers market is estimated to be USD 43.1 billion, with an expected compound annual growth rate (CAGR) of 12.6% from 2021 to 2026 (Figure 11).
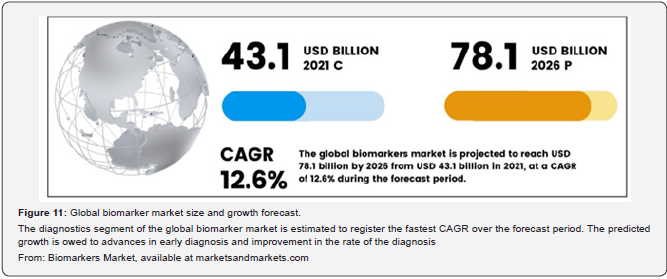
The key factors boosting market growth are:
i. An ageing population and increasing prevalence of chronic diseases.
ii. Advancements in the tools and OMICS technology used to discover and develop biomarker-based diagnostics.
Biomarkers play crucial roles in diagnosis, staging, treatment selection, and monitoring of diseases and their complications. Advances in OMICS-technologies have led to the identification of numerous candidate biomarkers with clinical potential. Integrating these biomarkers into evi-dence-based medical practice using emerging high-throughput technologies is essential for per-sonalized treatment and disease prevention. Additionally, biomarker research in autoimmunity and cancer is focused on identifying predictors of successful drug-free remission and uncovering molecular similarities between seemingly distinct diseases. Shifting focus from late-stage disease treatment to early-stage monitoring and prevention could transform medicine, with collaboration across academia, industry, and other sectors vital for realizing the full potential of biomarkers in personalized medicine.
- Research Article
- Abstract
- Introduction
- Biomarker categories in clinical practice
- Biomarkers and Personalized and Precision Oncology (PPO)
- Biomarkers of the next step generation
- Circulating tumor cells (CTCs) in clinical practice
- Сatalytic antibodies or abzymes
- Network-based biomarkers (NBBs)
- Biomarker-guided clinical trial design
- Future applications of biomarkers in healthcare
- Conclusion
- References
References
- https://www.medznat.ru/en/practice/medical-billing/precision-and-personalized-medicine-unlocking-the
- Zhan Xianquan, Zhou Tian, Cheng Tingting, Lu Miaolong (2019) Recognition of Multiomics- Based Molecule-Pattern Biomarker for Precise Prediction, Diagnosis, and Prognostic Assessment in Cancer.
- Ma JD, Lee KC, Kuo GM (2010) HLA-B*5701 testing to predict abacavir hypersensitivity. PLoS Curr.
- Faraoni I, Graziani G (2018) Role of BRCA Mutations in Cancer Treatment with Poly (ADP-ribose) Polymerase (PARP) Inhibitors. Cancers (Basel) 10(12): 487.
- Méndez Hernández R, Ramasco Rueda F (2023) Biomarkers as Prognostic Predictors and Therapeutic Guide in Critically Ill Patients: Clinical Evidence. J Pers Med 13(2): 333.
- García-Gutiérrez MS, Navarrete F, Sala F, Gasparyan A, Austrich-Olivares A, et al. (2020) Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front Psychiatry 11: 432.
- Chen CY, Wu KH, Guo BC, Lin WY, Chang YJ, et al. (2018) Personalized Medi-cine in Severe Asthma: From Biomarkers to Biologics. Int J Mol Sci 25(1): 182.
- de Nooijer AH, Pickkers P, Netea MG, Kox M (2023) Inflammatory biomarkers to predict the prognosis of acute bacterial and viral infections. J Crit Care 78: 154360.
- Kraus L (2023) Predictive Biomarkers: Shaping the Future of Personalized Medicine. Bi-omark J 9: 020.
- Rodríguez-Antona C, Taron M (2015) Pharmacogenomic biomarkers for personalized cancer treatment. J Intern Med 277(2): 201-217.
- Laigle L, Chadli L, Moingeon P (2023) Biomarker-driven development of new therapies for autoimmune diseases: current status and future promises. Expert Rev Clin Immunol 19(3): 305-314.
- Bolognesi ML, Gandini A, Prati F, Uliassi E (2016) From Companion Diagnostics to Theranostics: A New Avenue for Alzheimer's Disease? J Med Chem 59(17): 7759-7770.
- https://www.openpr.com/news/1930919/theranostics-market-to-surpass-20-billion-usd-by-2027-leading.
- Carini C, Fidock M, van Gool A (Eds.) (2019) Handbook of Biomarkers and Precision Medicine (1st). Chapman and Hall/CRC. 10.1201/9780429202872.
- Carrigan P, Krahn T (2016) Impact of Biomarkers on Personalized Medicine. Handb Exp Pharmacol 232: 285-311.
- Moore DC, Guinigundo AS (2023) Biomarker-Driven Oncology Clinical Trials: Novel De-signs in the Era of Precision Medicine. J Adv Pract Oncol 14(Suppl 1): 9-13.
- Bayes-Genis A, Voors AA, Zannad F, Januzzi JL, Mark Richards A, et al. (2018) Transitioning from usual care to biomarker-based personalized and precision medicine in heart failure: call for action. Eur Heart J 39(30): 2793-2799.
- Bayes-Genis A, Ordonez-Llanos J (2015) Multiple biomarker strategies for risk stratification in heart failure. Clin Chim Acta 443: 120-125.
- Fasano S, Milone A, Nicoletti GF, Isenberg DA, Ciccia F (2023) Precision medicine in systemic lupus erythematosus. Nat Rev Rheumatol 19(6): 331-342.
- Sato Y, Okamoto K, Kawano Y, Kasai A, Kawaguchi T, et al. (2023) Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives. J Clin Med 12(14): 4646.
- De Sa J, Carlson B, Caputo N, Vojta D, Sandy L (2013) Growth of molecular diagnostics and genetic testing in the USA, 2008-2011: analysis and implications. Per Med 10(8): 785-792.
- Purkayastha K, Dhar R, Pethusamy K, Srivastava T, Shankar A, et al. (2023) The issues and challenges with cancer biomarkers. J Cancer Res Ther 19(Supplement): S20-S35.
- Krystel-Whittemore M, Tan PH, Wen HY (2024) Predictive and prognostic biomarkers in breast tumours. Pathology 56(2): 186-191.
- Al-Dewik Nader, Younes Salma, Essa Musthafa, Pathak Surajit, Qoronfleh M (2022) Making Biomarkers Relevant to Healthcare Innovation and Precision Medicine. Processes 10(6): 1107.
- Chang Jason YH, Ladame Sylvain (2020) Diagnostic, prognostic, and predictive biomarkers for cancer p. 3-21.
- Sylvain Ladame, Jason YH Chang (2020) Bioengineering Innovative Solutions for Cancer. Academic Press.
- Pilla L, Alberti A, Di Mauro P, Gemelli M, Cogliati V, et al. (2020) Molecular and Immune Biomarkers for Cutaneous Melanoma: Current Status and Future Prospects. Cancers 12(11):3456.
- He Pei (2015) Personalized medicine: challenges in biomarker-related clinical trial design. Clinical Investigation 5:175-188.
- Sundarbose Kamini, Kartha Reena, Subramanian Subbaya (2013) MicroRNAs as Biomarkers in Cancer. Diagnostics 3: 84-104.
- Armand-Labit V, Pradines A (2017) Circulating cell-free microRNAs as clinical cancer biomarkers. Biomol Concepts 8(2): 61-81.
- Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, et al. (2020) miR-NAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 9(2): 276.
- Lawrence R, Watters M, Davies CR, Pantel K, Lu YJ (2023) Circulating tumour cells for early detection of clinically relevant cancer. Nat Rev Clin Oncol 20(7): 487-500.
- Davoudi F, Moradi A, Becker TM, Lock JG, Abbey B, et al. (2023) Genomic and Phenotypic Biomarkers for Precision Medicine Guidance in Advanced Prostate Cancer. Curr Treat Options Oncol 24(10): 1451-1471.
- Circulating Tumor Cells (CTCs). Available from: https://rarecyte.com/circulating-tumor-cells.
- Chaffey Ben, Silmon Angela (2016) Biomarkers in personalized medicine: Discovery and delivery. The Biochemist 38: 43-47.
- Silverstein A, Dudaev A, Studneva M, Aitken J, Blokh S, et al. (2022) Evolution of biomarker research in autoimmunity conditions for health professionals and clinical practice. Prog Mol Biol Transl Sci 190(1): 219-276.
- Zhao Daqun, Chen Jie, Hu Xiaoyue, Zhang Shujun (2022) Catalytic Anti-bod-ies: Design, Expression, and Their Applications in Medicine. Applied Biochemistry and Biotechnology 195: 1-27.
- Gabibov AG, Ponomarenko NA, Tretyak EB, Paltsev MA, Suchkov SV (2006) Catalytic au-toantibodies in clinical autoimmunity and modern medicine. Autoimmun Rev 5(5): 324-330.
- Suchkov SV, Gabibov AG, Gnuchev NV, Alekberova ZS (2001) The Distribution of DNA-Abzymes in Patients with Different Types of Systemic and Organ-Specific Autoim-mune Disorders. Russ J Immunol 6(3): 309-312.
- Suchkov Sergey (2021) A New Generation of Translational Tools designed to Monitor Multiple Sclerosis (MS) at Clinical and Subclinical Stages. Annals of Advanced Biomedical Sciences 1(4).
- Xuye Yuan, Jiajia Chen, Yuxin Lin, Yin Li, Lihua Xu (2017) Bairong Network Biomarkers Constructed from Gene Expres-sion and Protein-Protein Interaction Data for Accurate Prediction of Leukemia. Journal of Cancer 8: 278-286.
- Alfano C, Farina L, Petti M (2023) Networks as Biomarkers: Uses and Purposes. Genes (Basel) 14(2): 429.
- Lai TL, Lavori PW, Shih MC, Sikic BI (2012) Clinical trial designs for testing biomarker-based personalized therapies. Clin Trials 9(2): 141-154.
- Chauvie S, Mazzoni LN, O'Doherty J (2023) A Review on the Use of Imaging Biomarkers in Oncology Clinical Trials: Quality Assurance Strategies for Technical Validation. To-mography 9(5): 1876-1902.
- Veronika Medvedeva, Eric James Sorenson, Mariya Studneva, Noel Rose, Sergey Suchkov, et al. (2022) The Autoimmune Syndrome Through the Prism of Targeted AT-Mediated Proteolysis: Innovative Ideas, Philosophy, and Tools for Practitioners of The Next Step Generation. Am J Biomed Sci & Res 15(3).






























