A Solution for Ambient Storage of DNA
John H Phillips*
The Hospital for Sick Children, University of Toronto, Canada
Submission: July 11, 2023; Published: July 26, 2023
*Corresponding author: John H Phillips, The Hospital for Sick Children, University of Toronto, Canada. Email id: John.phillips@sickkids
How to cite this article: John H P. A Solution for Ambient Storage of DNA. Ann Rev Resear. 2023; 9(4): 555770. DOI: 10.19080/ARR.2023.08.555770
Abstract
The need for a solution for room temperature storage of DNA is well known. Studies were conducted to assess stability of EBV DNA using Daykin Molecular Solutions GeneGuardDNA (Georgia). All laboratory testing was done at Prime Laboratories in Van Nuys California. The objective is to demonstrate sample stability of EBV DNA during shipping, storage, and multiple freeze/thaw cycles. To achieve the objectives, the study was divided into two parts; Part 1 to demonstrate stability during shipping and multiple freeze/thaw cycles. Samples were labeled as P1-1 (Positive sample 1, P1-2 first freeze/thaw, P1-3 second freeze/thaw, etc.). In Part 1 the standard deviation between positive samples ranged from 0.08 CTs to 0.25 CTs with no gradual deterioration detected with more freeze/thaw cycles). The average was 0.17 CT’s which is within the instrument’s precision range. In the past we have had no instance of repeating the same extracted sample more than two times. In Part 2 we demonstrated stability during storage. 185 archived clinical samples stored at room temperature from 2013 through 2017 were randomly chosen. DNA was extracted from these samples and EBV DNA assay testing was performed. The results from the new test were compared to the original results obtained for the samples. Concordance and p values were calculated to show the effects of long-term storage on the results of the EBV DNA assay. Pearson correlation coefficient between the original and recent assay results was 0.9564 with a p value of p<0.001 indicating statistically significant correlation between the two assay results. EBV DNA showed no deterioration of sample Ct signal was observed due to Freeze/Thaw cycles and the results were stable across multiple cycles for a period of 6 years at room temperature.
Keywords: DNA; Stability; Ambient Storage
Introduction
Over a billion specimens of research materials, including DNA, RNA, cells, clones, tissue, organ products, blood, buccal, mucosal, vaginal, and other swabs, are stored globally, many of which are both critical and often irreplaceable for scientific research. Researchers within these laboratories have assembled very large collections of biological samples from clinical and field studies, stored in laboratories within institutes, commercial organizations, and University systems. More than 40,000 of these facilities exist within the US alone. These specimens represent enormous scientific and financial value for the researcher and these organizations. The collection and storage costs per sample range from a few dollars to as high as $10,000, The overwhelming majority of samples are stored in cold temperature systems and are susceptible to loss or degradation due to a variety of well-known circumstances [1].
Several advantages have been ascribed to room-temperature sample storage over the current cold-temperature-based storage. Perhaps the most significant benefit is around increased sustainability of biological research when samples are transitioned from cold freezers to ambient room temperature.
This process lowers energy consumption, reducing the carbon footprint (CO2 emission), lower the costs associated with equipment purchase and maintenance, lower material costs, and provide better utilization and optimization of lab space gained by retiring the cold freezers. A study by G. Jensen at Stanford University demonstrated in a Pilot Study demonstrated Stanford University could reduce its carbon footprint by eighteen thousand metric tons and save $16 million dollars in operating costs over the next ten years by transferring biological samples from frozen storage to room storage technology [2]. This equates to billions of dollars savings and millions of tons of carbon reduction globally and a dramatic reduction in risk of loss of critical Materials. DNA is particularly susceptible to degradation by hydrolytic and oxidative endogenous nucleases which if not prevented, break down DNA strands into fragments that may not be useful for PCR analysis [3-5]. While enzyme activity with the attendant DNA degradation may be limited by adjusting the ambient pH or salt concentration, cryopreservation is the preferred method of DNA protection. Deep freezing with dry ice or liquid nitrogen is often not convenient or feasible in an ambulatory setting such as in a physician’s office or at a remote clinic and is expensive and not environmentally sustainable.
Therefore, a need exists for a simple method to preserve specimens, at ambient temperatures, during transportation and long-term storage for eventual recovery of high quality, intact DNA. We demonstrate that Gene Guard DNA (Daykin Molecular Systems) is a nucleic acid transport media that is suitable for the interim preservation of nucleic acid(s) in the field, during transport to the laboratory from an ambulatory or remote clinic, and for general research laboratory storage. The solution consists of a combination of compounds including TRIS, EDTA, and NaCl. The concentration of EDTA chelates all divalent cations rendering Type I nucleases nonfunctional. The high concentration of NaCl not only causes cell creation and membrane disruption but also stops Type II nuclease activity completely and facilitates dissociation of proteins. This combination of high EDTA and NaCl concentration virtually stops all nuclease activity. The Tris buffer stabilizes and maintains the pH of the solution preventing degradation of the DNA at acid pH and prevents the precipitation of EDTA and NaCl.
Material and Methods
Studies were performed to assess stability of EBV DNA using Daykin Molecular Solutions Gene Guard DNA (Georgia). All laboratory testing was done at Prime Laboratories in Van Nuys California.
DNA Stability
The objective is to demonstrate sample stability of EBV DNA during shipping, storage, and multiple freeze/thaw cycles. To achieve the objectives, the study was divided into two parts; Part 1 to demonstrate stability during shipping and multiple freeze/ thaw cycles and Part 2 to demonstrate stability during storage.
Part 1: The samples were collected at the clinic and then preserved in the Gen Guard DNA during shipping, which ranged from 2 to 5 days. DNA extraction was performed 12 hours after receiving the samples, and the DNA was stored at 4oC. Five clinically positive samples were chosen for this study. After extraction the samples were each divided into 5 equal parts and they were subjected to up to 5 freeze/thaw cycles in the following manner: one part was run without freeze/thaw, another part with one cycle of freeze/thaw, the third part with 2 cycles of freeze/ thaw, etc. All sample parts were run simultaneously. The standard deviation was calculated between each sample part to determine if there was a variation and signal drop due to freeze/thaw cycles.
Part 2: 185 archived clinical samples stored at room temperature from 2013 through 2017 were randomly chosen. DNA was extracted from these samples and EBV DNA assay testing was performed. The results from the new test were compared to the original results obtained for the samples. Concordance and p values were calculated to show the effects of long-term storage on the results of the EBV DNA assay.
Result
DNA Stability
i. Part 1
The following are the results obtained from each set of Freeze/ Thawed samples: (Table 1)
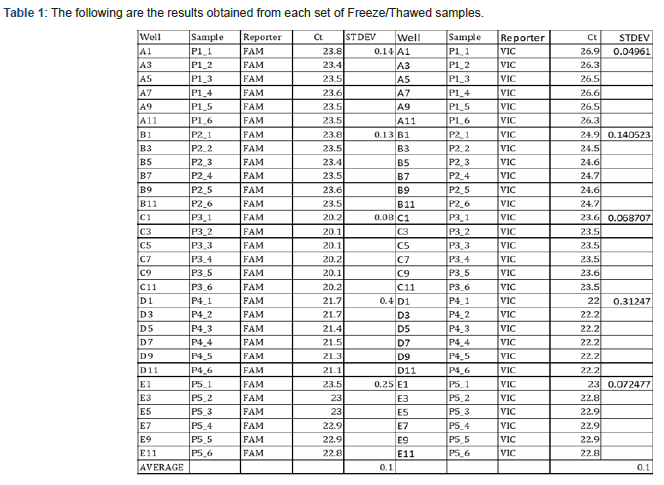
Samples were labeled as P1-1 (Positive sample 1, P1-2 first freeze/thaw, P1-3 second freeze/thaw, etc.). The standard deviation between positive samples ranged from 0.08 CTs to 0.25 CT’s with no gradual deterioration detected with more freeze/ thaw cycles). The average was 0.17 CT’s which is within the instrument’s precision range. In the past we have had no instance of repeating the same extracted sample more than two times.
ii. Part 2
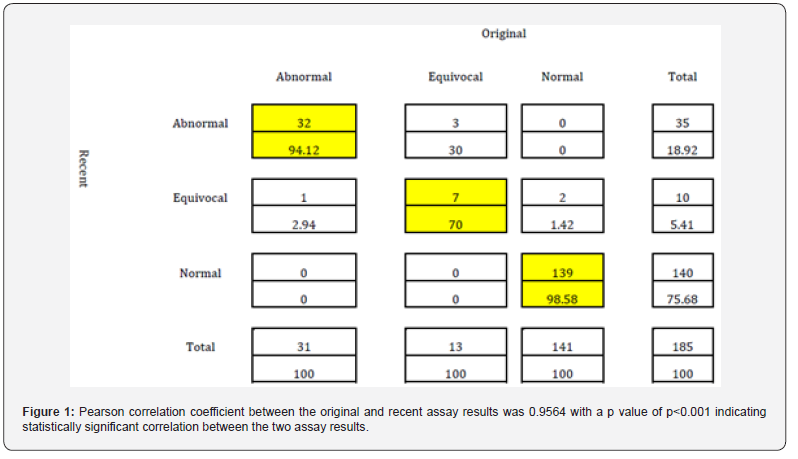
The following are the results obtained from the archived samples compared to the original sample results: (Table 2) When concordance was calculated between the archived and recent results, it was shown that there was high concordance between them. The following chart summarizes the concordant samples for each category with the concordance calculation underneath the numbers. The highlighted diagonal results indicate the concordance values for the Abnormal samples (94.12), Normal samples (98.58) and Equivocal samples (70.0) (Figure 1). Pearson correlation coefficient between the original and recent assay results was 0.9564 with a p value of p<0.001 indicating statistically significant correlation between the two assay results.

Discussion
A reliable and cost-effective system for DNA sample preservation invitro remains a significant challenge and opportunity for cost savings and environmental stewardship. Several methods exist commercially that are used to address these concerns. A new approach based on a formulation that uses mimicry of natural biological processes of DNA preservation has led to the development of novel synthetic formulations that have been shown to offer better stabilization, storage ability and shipping options for a wide range of nucleic acids. It mimics the natural biostability process of extremophile organisms in how they preserve their tissues and cells in a dry state for longer than 100 years and are still able to resume their normal function upon hydration. The original observation of this natural phenomenon was made as far back as 275 years ago by Antony van Leeuwenhoek, a Dutch microbiologist, who first coined the term ‘anhydrobiosis’, or life without water, to describe this natural phenomenon. EBV DNA showed no deterioration of sample Ct signal was observed due to Freeze/Thaw cycles and the results were stable across multiple cycles for a period of 6 years at room temperature..
Conclusion
No significant deterioration of sample results was observed over long term storage of up to 6 years of the patient samples for the EBV DNA at room temperature.
Conflict of Interest
i. John Phillips is the Chief Medical Officer for Daykin Molecular Systems. He is an Associate Professor of Surgery at The University of Toronto and has extensive publications and experience in handling regulatory issues on launching medical devices in North America. Dr. Phillips was involved in the development and FDA approval of NPScreen which involves room temperature storage and transportation of cells for the molecular diagnosis of nasopharyngeal carcinoma.
References
- Iyengar GV, Subramanian KS, Woittliez Joost (1997) Elemental Analysis of Biological Samples: Principles and Practices, New York: CRC Press p 82.
- Jensen G Fact Sheet: Room temperature storage saves energy.
- Shikama K (1965) Effect of freezing and thawing on the stability of double helix of DNA. Nature 207(996): 529-530.
- Clement O (2009) Biological sample storage and management. Lab Manager p. 26-29.
- Dutton G (2005) Thinking outside the ice box of DNA. The Scientist 19(14): 28.






























