Evaluation of Boar’s Semen Production and Characteristics for Artificial Insemination
Méndez PN1*, Galicia DJA1, Méndez PNN1, Vázquez FF1, Campos RM1 and Méndez MM1
Faculty of Veterinary Medicine and Zootechnics, Tecamachalcom Meritorious Autonomous University of Puebla Mexico
Submission: July 02, 2023; Published: July 12, 2023
*Corresponding author: Méndez PN, Faculty of Veterinary Medicine and Zootechnics, Meritorious Autonomous University of Puebla Mexico. Email id: nmp63mx@gmail.com
How to cite this article: Méndez PN, Galicia DJA, Méndez PNN, Vázquez FF, Campos RM, et al. Evaluation of Boar’s Semen Production and Characteristics for Artificial Insemination. Ann Rev Resear. 2023; 9(4): 555766. DOI: 10.19080/ARR.2023.09.555766
Abstract
The objective of this work was to evaluate the production and characteristics of boar semen from different genetic lines used for artificial insemination, for which 19 boars were used: LM100 (n=4); LM200 (n=2); LM300 (n=2); LTP27 (n=4); LTH1 (n=2); LTB1 (n=3) and LTB37 (n=2) for two and a half years; the variables evaluated were Ejaculate Volume, Motility %, Temperature, Sperm Concentration, Collection frequency, Season of the year, Age and Number of doses. The general averages of the results obtained were: semen volume 263.64 ± 3.59, Motility 89.99 ± 0.20, Ejaculate temperature 37.12 ± 0.03, Dose x ejaculate 19.43 ± 0.36, Winter season, semen volume 263.69 ± 3.59, Spring Motility 90.81 ± 0.43, Autumn temperature of the Ejaculate 37.12 ± 2.74, Spring number of doses (300 x 109) ; 19.43 ± .35, in relation to the Genetic Lines in which there was a significant difference with respect to the evaluated variables were in Volume it was LTP-27 with 328.74 ± 7.97 mL (P <0.05), in % Motility it was LTH-1, 91.81 ± 0.52 (P<0.05), in Ejaculate Temperature The LTB-1 with 37.70 ± .09 (P>0.05), in dose was the LTP-27, 23.15 ± 0.64 (P<0.05), the behavior by season of the year and your variables.
The general mean of ejaculate volume in winter was 271.02 ± 8.40 (P<0.05); The % motility in spring was 90.81 ± 0.43 P<0.05, in temperature it was 37.21 ± 37.21 (P<0.05), the average longest collection days in spring was 10.12 ± 0.34 (P<0.05); when comparing the Volume of the ejaculate by maternal genetic line (LM100,200,300) and Terminal (LTP27, LTH1, LTB1 and LTB37) it was 238.75 ± 3.58 Vs. 279.96 ± 5.31 (P<0.05), Motility % 91.23 ± 0.26 Vs. 89.18 ± 0.27 (P<0.05), sperm concentration 22.71 ± 0.545 Vs. 22.19 ± 0.42 (P>0.05), Ejaculate temperature 36.91 ± 0.03 Vs. 37.25±0.03 (P<0.05), Sperm per ejaculate 5.3 x 109 ± 1.3 x 109 Vs. 6.1 x 109±1.4 x109 (P<0.05), Number of doses 11.16 ± 0.27 Vs. 16.55±0.31 (P<0.05), Sperm per dose 370.82 ± 5.11 Vs. 331.78 ± 3.15 x 109 (P<0.05) , Dose (300 x 109), 17.79 ± 0.45 Vs. 20.35 ± 0.47 (P<0.05) Maternal Vs. Terminal line respectively in the variables described above. When evaluating the age of the stallions, the results were: Ejaculate volume 263.64 ± 3.58 (P<0.05), % Motility 89.99 ± 1.98 (P<0.05), Concentrated sperm 22.40 ± 0.33 (P>0.05), Temperature 37.119 ± 0.02 (P<0.05), Sperm per ejaculate 5.8 x 109 ± 1.0 x 109 (P<0.05), Number of doses 14.42 ± .239 P>0.05, Sperm per dose 347.24 ± 2.86 (P<0.05), Dose 300 x 109 19.34 ± .342 P<0.05 with a mean age of 26.5 months for the maternal line. It is concluded that the Terminal genetic lines have better performance in the parameters, when compared against the maternal lines when the production and characteristics of the semen of the boars used for artificial insemination were evaluated. Likewise, age and season of the year are determining factors.
Keywords: Artificial Insemination; Boar´s Semen; ANOVA; Morphoanomalies; Spermatozoa; Spermatozoon Membrane
Abbreviations: PAI: Porcine Artificial Insemination; AI: Artificial insemination
Introduction
Porcine artificial insemination (PAI.) is a first-generation reproductive biotechnology that consists of depositing semen in the genital tract of the female by means of instruments Decuadro [1]. Swine artificial insemination plays an important role for genetic improvement in animals, considering the use of information to improve genetic composition according to important economic characteristics Dion [2]. This integration is through the use of statistical models and computational methodologies as well as proven reproductive technologies to select and disseminate genetic improvement to large populations Decuadro [3]. During the last 10 years the use of Artificial insemination (AI) has increased enormously. Martínez [4]; Raath [5]. It is estimated that currently, of the 76 million sows in the world, more than 90% are inseminated. The countries of the European community lead the list of the use of A.I. with 95.7% of inseminated sows, with France, Finland, and Spain being the main ones. In the Pacific region and Asia, 69% of the sows are inseminated while in Latin America the percentage of use is 60%, particularly in Mexico they are at 85% AI Salazar [6]. The P.A.I. are a joint series of semen collection, treatment and conservation techniques as well as insemination per se Gosálvez [7]. Their effectiveness can be estimated through the percentage of fertility and births as well as the size of the litter Pallas [8]. These characteristics have made the pig sector react, producing a progressive evaluation in the use of the technique, being increasingly productive and effective, guaranteed with important biosecurity measures the sanitary quality of the doses and together with the increase in similar doses Córdoba [9]. The boars used for P.A.I. are selected through the BLUP (Best linear unbased prediction), the best unbiased linear prediction Batista [10], which considers all the information from all the relatives of an individual to calculate an estimated breeding value Becerril and Rocha [11]. With what allows to compare individuals or populations. To currently know the biotechnological developments that have made it possible to mark genes in the DNA associated with quantitative characteristics associated with growth, back fat and carcass characteristics in pigs.
Information that is taken into account in addition to the selection criteria that has allowed the development of the selection assisted by genetic markers. With which the precision and intensity of selection is increased and therefore the genetic progress, Stephano [12] through A.I. since obtaining good results in AI in pigs depends on the level of ability to develop the techniques collection, dilution, conservation, advantages it presents Jonson [13]: reduction in the number of boars on the farm, use of boars of high genetic quality allowing a general improvement of the herd, maximum exploitation of management in groups or lots, obtaining % fertility equal to or greater than those obtained in natural mating, facilitate handling by reducing time and work / mating, better control of semen quality and better sanitary control that allows obtaining reciprocal protection of male and female reproductives, avoiding contamination of females by the intermediate of infected males or preventing the contamination of the males during intercourse in the case of infected females Stephano [12]; Becerril and Rocha [11]. However, the real cause of the current increase in P.A.I. in the world is polyvalent: better knowledge of the physiology and management of sows and semen, better equipment, better extenders and a greater demand from consumers regarding the quality of the pork produced. However, the fact that the advantages of the P.A.I. Pallas [8]; Córdoba [9], far outweigh the drawbacks, does not mean that the use of this biotechnology is easy; In this sense, we have to remember that the era of the use of P.A.I., in certain countries, has initially failed due to its incorrect application or the existence of problems or management deficiencies that cannot be solved by the simple use of A.I. (Flowers and Esbenshade [14]; PIC [15]. Therefore, the objectives of this work were to evaluate the production and characteristics of the semen of the different genetic lines of the boars used for A. I. in a private company, as well as to know the ejaculate properties such as volume, motility, semen temperature, sperm concentration, collection frequency Kubus [16], boar age and season of the year.
Materials and Methods The work was carried
The work was carried out in the artificial insemination center of the Super Gen pig genetic improvement nucleus farm located in Quecholac Puebla. Geographically, the municipality of Quecholac is located in the central eastern part of the state of Puebla (INEGI, 1998). Its geographical coordinates are the parallels 18º 49’ 18” and 19º 00’ 18” north latitude and the meridians 97º 34’ 42” and 97º 44’ 54” west longitude. Its borders are to the north with Felipe Ángeles and San Juan Atenco, to the southeast with Palmar de Bravo, to the east with Ciudad Serdán (Chalchicomula de Sesma) and to the west with Acatzingo and Tecamachalco (INEGI, 1992). It has an area of 163.29 km2 that places it in 83rd place with respect to the other municipalities in the state. Localities that constitute it: Palmarito Tochapa, San Simón de Bravo, Tuzoapan, La Compañía, Guadalupe Enríquez, Santa Catarina Villanueva, Francisco I. Madero INEGI [17]. With an average altitude of 2,080 meters above sea level. Subhumid temperate climate with summer rains, soils that exist: Xerosol, cambisol, litosol, regosol, feozem. The federal highway Mexico-Córdoba crosses the municipality from east to south. A state highway heading south leaves the municipal seat and connects with the highway to Tehuacan-Tecamachalco INEGI [18].
Animals
Nineteen stallions from different genetic lines were used: LM- 300 (n=2), LM-200 (N=2), LM-100 (n=4), LTP27 (n=4), LTH1 (n=2), LTB1 (n=2), LTB137 (n=2). The diluent used for this research was MR-A (long duration), for a period of 2.5 years, and the productive characteristics of the boars’ semen were determined.
Experimental design
An experimental design of repeated observations was used, the data was analyzed through a simple ANOVA and a Tukey test for multiple comparisons, in the statistical program SPSS v. 13 (2005).
Statistical Model
Yijk = M+ tj+Si ( j) + Pk + (TP) jk + Eijk
Where:
Yijk= Measurement in Subject i, of treatment j, in period K
M= General Mean
Tj=Effect of treatment j; j =1…..t
If ( j) effect of subject i, where treatment j .
Inter-subject error i = 1……s
Pk= Effect of period k; k= 1……..p
(Tp) jk = Effect of the interaction of Tx j and period k
Eijk= Inter-subject error (in fact, it supposes no interaction (Pxs)
Inside the Ti).
Procedure
Semen collection
Semen is obtained using a riding dummy and double gloves, cleaning the sides of the stallion and the preputial diverticulum, then pressing the penis to stimulate the ejaculate Loula [19]. It is collected in a tempered thermos, which has filter paper to avoid tapioca or smegma. Once the semen has been collected, it is weighed to determine the total volume. A mercury thermometer is introduced into the semen to know the temperature of the ejaculate Ubeda et al. [20]. The semen used was from the 2nd spermatic or rich fraction (fraction that comes from the epididymis and that contains 70% of the spermatozoa in the ejaculate, representing 30-50% of the total volume Donald [21].
Motility
A slide is heated to a temperature of 37 °C, then a drop of semen is taken with a Pasteur pipette (Kubus, 2000), the drop of semen is placed on the slide and covered with a coverslip, the percentage of motility is evaluated. Progressive analysis of the spermatozoa in the light microscope, with the 10X weak dry objective and the 40X strong dry objective Flowers [14]; Loula [19].
Concentration
Using a graduated pipette to 1 cubic centimeter, a 1:100 dilution is made in a formulated saline solution, one centimeter of pure semen is added, making up to 100cc Burker [22]. A drop of semen is taken and deposited in the reticulum of the Burker chamber. Dead spermatozoa are counted in 40 frames with the 40X strong dry objective Decuadro [1]. The counted spermatozoa without morphoanomalies are added Burker [23]. Knowing its concentration, we calculate the number of doses that can be obtained from that ejaculate at a concentration of 3 x 109 to obtain good quality semen Kubus [16]; Technical Department of Minitube Mexico [24]; Flowers [25]; Roozeboom [26].
Dose calculation
Number of doses (N) = (A) x 10,000 x 1,000 x (V) 3x109
Simplified formula.
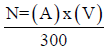
Bürker chamber concentration
i. 40 little squares =A
ii. A x 100 =mm3
iii. A x 100000 x 100=CC
iv. A x 107 spz/CC
(V) = Volume of the ejaculate in CC.
(A) = Sperm (SPZ). Counted in 40 squares.
(C) = Number of spermatozoa.
(C) = In mm3 = (A) x 10,000 = SPZ /mm3.
(C) = In CC = (A) x 10,000 x 1,000 = SPZ / CC.
(C) Total = (A) x 10,000 x 1,000 x (V) = Total SPZ in the Ejaculate.
Dilution
The MR-A extender is heated in a thermoplate or in a water bath, the temperature is equalized to that of the semen collected, the mixture of semen and extender is homogenized, and motility is observed again Fragoso [27]; Escobedo [28].
Packing
It is carried out in a plastic (cochette bag) which contains identification of the stallion, genetic line to which it belongs, expiration date, and 100 ml of semen Flowers [25]; Kubus [16].
Temperature
It affects the quality of the semen since it is particularly sensitive to thermal changes, so it is vital to keep it between 18 °C, to avoid fluctuations in temperature. The temperature of the semen is gradually reduced for 2 or 3 hours for its conservation Kubus [16]; Goss, 2003). The decrease in temperature below 14 °C causes alterations in the spermatozoon membrane, affecting its fertilizing power Decuadro [1]; Pursel and Johnson [29]. Temperatures above 20°C do not lower sperm metabolism or stop bacterial growth, which greatly reduces the useful life of semen Conejo [30]; Johnson [13]; Bertrand [31].
Transport and conservation of semen
The semen is transported through insulating material such as Styrofoam boxes that prevent temperature variation of the semen. Flowers [25]; Roozeboom [26]. Doses are stored at 18ºC.
Variables to Evaluate
a) Volume of the ejaculate.
b) Motility.
c) Semen temperature.
d) Sperm concentration.
e) Collection frequency.
f) Number of doses
g) Season of the year.
h) Age of the boar.
i) Genetic line of the stallion.
Results and Discussion
The results are described following the variables evaluated:
Ejaculate volume
In Table 1 The results of each Boar are presented, noting that the highest volume corresponds to the LTP-27 genetic line, and the lowest volume is presented by the LTB-37 line. As the results obtained in this work can be observed, the volume produced by the different genetic lines and the collection rhythms, it is observed that the best genetic line was LTP-27 with a total ejaculate of 328.74 ml. In a study carried out by García Ruvalcaba et al., (1996), they obtained an average of 310.5 ml of ejaculate, with an interval of 5 days between collections. Being this lower than the present study, this is possibly due to the fact that in this work the semen collection was 8.59 days on average. In another investigation carried out in (1998) by Salazar, he found a volume of 207 ml with a collection interval of 3 days, being less than what was found in this study, differing with 121.74 ml in 5.59 more days. Donald [32], obtained 291 ml of ejaculate with an average of 6 days between collections. Which means that the longer the time between collections, the greater the volume of ejaculate (Table 2).
Motility
In relation to sperm motility between genetic lines, it was observed that LTH-1 had a (91.82%) and LM-100 (91.77%) being the highest, as can be seen in Table 1, the genetic line that had the lowest motility was LTB. -1 with (87.16%) (p<0.05) compared to those with the highest motility. Salazar [6] obtained 87.07% Motility in their samples studied, which means that in this study the performance in this variable was better when compared with the results obtained by before Salazar, this may be due to the Hybrid Vigor of the genetic lines studied.
Semen temperature
The temperature of the semen influences according to the genetic line, determining that the highest temperature of the ejaculate recorded for LTB-1 with (37.7 ºC) and the lowest for LTB-37 with (36.83 ºC) see Table 1, we determined this according to animal temperament and genetic line.
Dose 1 x 109
The number of doses obtained by the different genetic lines was analyzed, where it was observed that the best genetic line is LTP-27, with 23.15 on average, compared to the one that gave the lowest number of doses produced, LTB-37, with 12.18 on average Table 1. According to the number of doses produced, they coincide with the results obtained by Salazar [6]; Stephano [12], where they report 24.27 doses and 20 doses respectively, these results are attributed to the similarity in terms of the elaboration process of the seminal doses and in terms of the concentration in the dilution.
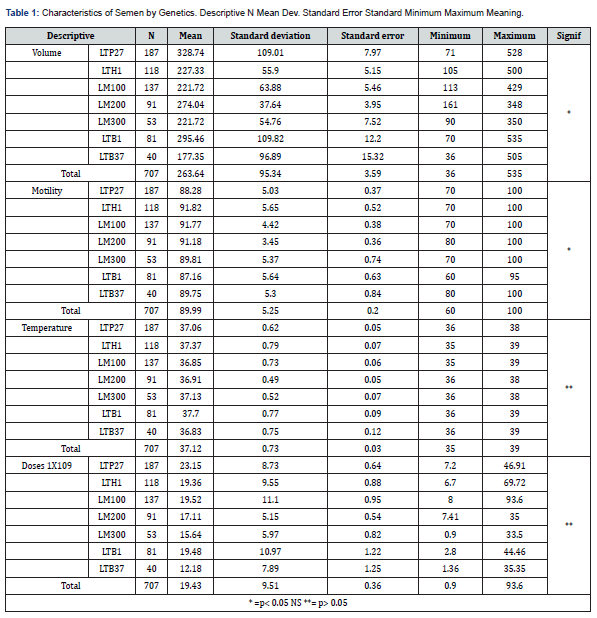
Volume by Station
Taking into account the season of the year, it was observed that the largest volume is produced in winter, with 255 ml. And the season with the lowest volume is in summer, the cause being the high temperature. As can be observed the results obtained in the present work where the volume produced by the different genetic lines, agree with those observed by Cameron, in (1992) found 367 ml in winter and in summer 274 ml. Also, Salazar [12] demonstrated; that in winter an ejaculate of 207 ml is obtained and in summer 188 ml.Motility by Season
It demonstrates the motility percentages found according to the season of the year, observing that there is a greater motility in all the genetic lines during the spring, it is also observed that there is a lower percentage of motility during the autumn. As can be seen, the results obtained where the motility produced by all the genetic lines agree with what was observed by Salazar [12] who found 87.07% motility in spring, also obtaining 82.31% in autumn.
Collection Interval Days by Station
Demonstrates the days of collection of stallions, during the seasons of the year, noting that there are a greater number of days in spring and summer, also noting that during autumn and winter there is the lowest range that exists in spermatic ejaculations by stallions. In the same way Nazare Torres observed in [33] who found similar results to the intervals of days between ejaculates obtaining the same similarity of the doses produced by the different genetic lines, autumn - 7 days (19 days) and spring - 5 days (20 doses).
Semen temperature by season.
The semen temperature of the different lines was analyzed during the seasons of the year and it was observed that the season where the temperature is higher is during the autumn months, there is also a lower semen temperature of the genetic lines during the summer.
Seminal Doses Produced by Season
The number of doses produced from the different genetic lines during the seasons of the year is analyzed, observing that in spring there is a higher production of doses as well as low production of doses during the autumn months. According to the results obtained, it was the record of the doses produced by the different genetic lines during the season of the year as shown by the reports of Stephano [12]; Salazar [6], 21 days in spring - autumn and 19 days and 19.7 days spring -16 days autumn.
Ejaculate Volume by Maternal and Terminal Genetic Lines
As we can see the results obtained in this work, where the volume produced by the different genetic lines was taken into account, we demonstrate that the best genetic line is the terminal one, obtaining a greater ejaculate. In a study carried out by Stephano [12]: he obtained a similar result of 265.25. ml., in another investigation carried out (1998) by Salazar found results of 207 ml. We attribute the similar results to the method of semen collection.
Motility between Lines
It represents the variables that the motility suffers between the genetic, maternal and terminal lines where it is observed that the maternal line has the highest motility with 91.23%. According to the results obtained based on the motility percentage, they coincide with those observed by Donal (1998), where he observed 90.75% motility in the semen.
Sperm concentration by lines
Analyzing the number of counted concentrated spermatozoa, we determined the amount of doses produced per ejaculate, between genetic lines, observing that maternal line boars have a higher sperm concentration, it is also observed that there is a lower sperm concentration in terminal line stallions. In a study carried out by Stephano [12], a result of 24.19 counted spermatozoa was obtained. Being this similar to the present study, this is possibly due to the way of interpreting the sperm count in the Burker chamber.
Semen Temperature between Lines
Semen temperature was analyzed between maternal and terminal lines. Where it was determined that the highest recorded ejaculate temperature is in the terminal line, this could be observed according to the genetic temperament of the boars, and there is also a lower semen temperature of the terminal line stallions. Flowers [25] observed in the same way, where he obtained results similar to those obtained at 37.00º C.
Sperm per ejaculate between genetic lines
According to the results obtained in the number of spermatozoa per ejaculate, we can define that the terminal line has a greater production of sperm. As can be seen, the results agree with those obtained by Donal, (1995), where it was obtained 60, 259, 346, and 382. For ejaculate attributed to the similarity in terms of the semen collection process.
Number of doses
The number of doses obtained between the maternal and terminal lines is analyzed, where it was observed that the best genetic line is the terminal producing a greater number of doses, also showing a lower number of doses produced by the maternal ones. As can be seen the results obtained by Batista [10], obtained 18 doses per ejaculate. Another study by Donald [32] obtained 17.53 doses.
Sperm per dose
We demonstrate the number of spermatozoa contained in each dose produced by genetic line where we found that the maternal stallions have a higher concentration of sperm in each dose, it is also observed that the terminal line has a lower number of spermatozoa concentrated per dose. As we can analyze the results obtained, they agree with those observed by Salazar [6] & Stephano [12], where they obtained 387.34 and 321.45 respectively, the similar results obtained are attributed to the way of processing the semen and the demand for doses within the farm.
Dose 3 x 109
The number of doses obtained per ejaculate between the maternal and terminal lines is analyzed, where it is observed that the best genetic line is the terminal, producing a greater number of doses, also demonstrating that the maternal line produces fewer doses. According to the results obtained in the number of doses produced, they coincide with the results obtained by Nazare Torres [33], obtained 20 doses. In other investigations carried out by Stephano [12] & Salazar [6], 21ds. And 19.7 days. Similar results are attributed according to the standard preparation used for the elaboration of doses in terms of sperm content.
Volume by age
According to the results obtained in terms of volume produced by the different genetic lines. We determined that a higher semen production is reached during the age of 30-41 months, where an X= 263.64 ml is obtained of volume. In a study carried out by Stephano [12], it was observed boars between 24-38 months of age where a volume X of 265.25 ml is obtained. The similarity of the results is attributed to different factors such as: Semen evaluation, age, breed, type of accommodation, season of the year, free or controlled environment, health, nutrition and collection rhythms.
Motility during age
It represents the variables that the motility of the different genetic lines suffers during different ages, where it is observed that there is a greater motility between 89.99% at 17-28 months of age. Likewise, there is a minimum motility variance during the other months of age. In the same way I observe Salazar [6], 90.54% of motility between 15-27 months of age. We attribute the similar results to the process of assessing semen quality.
Concentration of waits by age
The number of concentrated spermatozoa counted inside the burker chamber of the boars during different ages is analyzed, observing that there is a higher concentration of spermatozoa during 9-33 months of age where a concentration X=22.40 is found. In an investigation carried out by Stephano [12], found a sperm concentration X= 24.19.
Semen temperature
The temperature of the semen of the different maternal and terminal genetic lines was analyzed during different ages and observing that there is a higher temperature of the semen between 7-25 months of age of the boars where a temperature X = 37.11 is found. In a study carried out by Flowers [25], he found an ejaculate temperature of 37.00º C, at an age of boars between 9-24 months.
Sperm by ejaculate and age between genetic lines
As we can observe the results obtained in this work where the age of the stallions was taken into account, spermatozoa contained within the total volume of semen, it was observed that there is a greater quantity of spermatozoa during the 9-40 month of age, where there is a total sperm production in the ejaculate of 58,000, 000, 000. As can be seen, the results agree with those obtained by Donal (1995), where he obtained 60259346382, at an age of the stallions of 11-43 months, the similarity of the results is attributed to the semen collection process and age of the boars.
Number of doses by age
The number of doses produced at different ages of the different genetic lines is analyzed, where it is observed that the highest number of doses produced is during the 14-41 month period, obtaining an average of 14.42 doses. As can be observed the results obtained in an investigation by Batista [10], where he obtained 18.0 doses, at 12-43 months of age of the boars, another study carried out by Donald [32], obtained 17.53 doses. With an age of stallions from 13-39 months. The similarity of the results is attributed to the age of the stallions, the way of elaboration of doses based on their contained sperm concentration.
Sperm by dose and different ages of stallions
As we can observe the results obtained in this investigation where the number of spermatozoa contained per dose and the age of the boars were taken into account, it is shown that there is a higher concentration of spermatozoa X= 347.24 during 9-40 months of age depending on the number of doses and time of use of the same. In a study carried out by Nazare Torres [33], obtained a sperm concentration per dose of 300.00, at an age of the boars between 11-42 months. Similar results can be attributed to the way semen quality is assessed and the age of the stallions used.
Dose 3 x 109 and age of the boars
The number of doses produced per ejaculate and age of the stallions are analyzed where it is observed that there is a higher production of 19.34 doses, at an age of 9-41 months. According to the results obtained in the number of doses produced and the age of the stallions, coincide with the results obtained by Nazare Torres [33], where obtained 20 doses, in boars with an age of 11- 41 months. In other investigations carried out by Stephano [12] & Salazar [6], 21days and 19.7 days, in stallions aged 12-48 months and 10-45 months. Similar results are attributed according to the standard preparation used for the preparation of doses in terms of sperm content and age of the boars used [34-89].
Conclusions
a) Ejaculate volume, by season of the year, age of the boar, genetic line was for LTP-27 and the lowest volume was for LTB-37.
b) Motility, by season of the year, age of the boar, genetic line was for LTH-1 and the one with the lowest % was for LTB-1.
c) The highest temperature of the semen by season of the year, age of the boar, genetic line was for LTB-1 and the lowest belonged to LTB-37.
d) Sperm concentration by season of the year, age of the boar, genetic line was for LTH-1 and the lowest concentration was for LTB-37.
e) Collection frequency by season of the year, age of the boar, genetic line had no significant differences (p≤0.05).
f) Number of doses per season of the year, age of the boar, genetic line, no significant differences were found (p≤0.05). Finally, it can be concluded that the longer the time between collections, the greater the volume of ejaculate, the better concentration, better motility, and the greater number of doses.
References
- Decuadro H (1999) G Memoria Avances en la Inseminación Artificial Porcina de Curso de IAP Y Manejo Reproductivo del Cerdo; agosto 2-3, Tehuacan (Puebla) México p. 1-15.
- Dion N (1999) Nueva Tecnología Para el Mejoramiento Gené Los Porcicultores y su Torno 11: 50-51.
- Decuadro H (2001) G Los Beneficios de la IA, Acontecer Porcino Diciembre – Enero 9(46): 22-23.
- Martínez EA (1999) Posibilidades Practicas del Sexaje de Espermatozoides en la Especie Porcina. VI Simposium Internacional de Reproducción en Inseminación Artificial Porcina. Madrid Spain pp. 55-62.
- Raath D (1999) Resent advancements in male and female pig reproductió II Congreso Ibérico de Reproducción Animal. Lugo, Spain pp. 337-359.
- Salazar PMA (1998) Problemática de la Inseminación Artificial en Zonas Á Memoria del V Simposium Intrernaciomal de Reproducción e Inseminación Artificial en Porcinos mayo 4-6 León (Guanajuato) México, AMVEC pp. 238-248.
- Gonsálvez LF, Vidal A, Valdelvira J, Alonso J (2001) Congelación a Distintas Temperaturas de Semen Porcino Heterospérmico de Baja Calidad. Revista Cerdos (Swine) 42: 6-10.
- Pallas R, De Alba C (2003) Impacto de Nuevas Tecnologías de Inseminación Artificial Sobre la Administración de un centro de inseminación Artificial. Revista Credos 64(65): 19-22.
- Córdoba IA (2003) Biotecnología de la Reproducción Porcina. Revista Acontecer Porcino 12(63): 45-48.
- Batista L (2002) Manejo de Centros de Inseminación artificial en climas cá Revista Acontecer Porcino 54: 58-63.
- Becerril AJ, Rocha CG (2004) Nuevas Tecnologías Reproductivas para el Mejoramiento Gené Revista Acontecer Porcino 12(64): 22-28.
- Stephano HA, y Castro GE (1998) Implementación de un Programa de Inseminación Artificial. En un Complejo de 30,000 Hembras en Nebraska. USA. Memoria. V Simposium Internacional de Reproducción e Inseminación Artificial en Porcinos, Mayo 4-6 León (Guanajuato) México, AMVEC p. 43-44.
- Johnson L (1998) The AI Newsletter from Minitube of America 2(2): 6-16.
- Flowers LW, Esbenshade KL (1993) Optimizing management of natural and artificial matings in swine. J Reprod fertil Supplement 48: 217-228.
- PIC México Visión Técnica (1997) Departamento de producció Inseminación Artificial en Granjas. Articulo April de 1(6).
- Kubus SA (2000) Inseminación Artificial Porcina.
- (1996) Instituto Nacional de Estadística Geografía e Informática, Anuario Estadístico del Estado de Puebla.
- (1990) Instituto Nacional de Estadística Geografía e Informá XI Censo General de Población y Vivienda.
- Loula T (1997) AASP Quebec, Canada in: Common mistakes and solutions in the breeding area. Swine reproduction Workshop 10: 19-28.
- Ubeda EJL, Suñén CLA (1995) Consideraciones Sobre el Manejo de la Hembra en la Inseminación Artificial. Revista Acontecer porcino 111(14): 8-17.
- Donald GL (2000) Recomendaciones de Inseminación Artificial Razones Bioló Acontecer Porcino 8(41): 58-60.
- Burke P (2000) productivity assessment of liquid boar semen usage. En: IV International Conference on Boar Semen Preservation. Editado por IA. Johntson y HD, Guthrie, Maryland, EU pp. 149-150.
- Burker P (1996) Obtención de los Beneficios de la inseminación artificial en porcinos. Memoria del VII Seminario internacional para clientes PIC, Mayo (23): 1-33.
- (2000) Departamento Técnico de Minitube México Integridad de la protuberancia Acrosomal para evaluar la calidad espermá Los Porcicultores y su Entorno 32: 3-13.
- Flowers LW (1998) Managemente of the Boar user For Artificial Inseminatió Memoria de V Simposium Internacional de Reproducción e Inseminación Artificial en porcinos, León (Guanajuato) México, AMVEC (4-6): 77-81.
- Roozeboom kJ (2001) Evaluación de la calidad del semen porcino I. Revista Cerdos (swine) 39: 16-18.
- Fragoso VMA (1993) Manual de Inseminación Artificial Porcina (tesis de licenciatura) México (DF). Facultad de Medicina Veterinaria y Zootecnia Universidad Nacional Autónoma de México.
- Escobedo GM (1997) Inseminación Artificial. Desarrollo Porcicola 4: 7-10.
- Pursel VG and Johnson LA (2000) In: Fertility comparison of boar semen in two extenders. J Anim Sci 35(1123) (abst).
- Conejo NJ, Becerril AJ (1996) Ortega GR Diluyente de Corto Periodo de Almacenamiento Utilizados en la Conservación del Semen Porcino. II Jornada en.
- Bertrand J (1998) Microscopic Sperm Morphology: A Tool for Semen Quality Analysis. Spermnotes the AI Newsletter from Minitube of America 2(2): p. 17-18.
- Donald GL (2005) Current Boar Stud Management Practices. Boar Stud Issues. Seminar Numero 5. American Association Swine Veterinarians Toronto, Notario, US p. 11-24.
- Nazare TSLM (1998) Inseminación Artificial en Brasil. Memoria de V Simposium Internacional de Reproducción e Inseminación Artificial en Porcinos Mayo 4-6 León (Guanajuato) México, AMVEC pp. 33 - 41.
- Bazer FW, Thatcher ww, Martinat-Botté F, Terqui M (1998) Sexual maturation and morphological development of the reproductive tract in Large-White and prolific Chinese Meisham pigs. Journal Reprod Fertil 83(2): 723-728.
- Becerril AJ (2001) Desarrollo de los Programas de Inseminación Artificial y Manejo del Semen. Revista Cerdos (swine) 46: 32-34.
- Boar semen preservation II, 1990 In Comercial use of swine AI worldwide, 299-333, Beltsville, MD, USA.
- Care J, Behan J (1996) Novel methods of collection and processing of boar semen in “Al Vets” Escocia p. 1-6.
- Castañeda MJ, Becerril AJ, Sosa FC, Méndez LA (1998) Aportes Prácticos Para Programas de Inseminación Artificial en Granjas Porcinas. Memoria de V Simposium Internacional de Reproducción e Inseminación Artificial en Porcinos. Mayo 4-6; León (Guanajuato) México, AMVEC pp. 226-229.
- Castañeda MJ,Domínguez SF (1999) Tres Sitios, en Centros de Inseminación Artificial Mejoramiento Gené Los Porcicultores y su Entorno 8: 28-30.
- Castillo MJ (2002) Nutrición Para la Reproducció Memoria del 1V Simposium Curso de Reproducción e Inseminación Porcina. Mayo 28-30 Tlaxcala, Tlax.
- Cameron RDA (1992) Efectos del estrés por calor en la fertilidad del verraco. Revista síntesis porcina 11(4): 36-38.
- Conejo NJ (1990) Manual de Inseminación Artificial de ganado porcino con semen diluido. UMSNH Morelia Mich, Mé
- Córdova J (1994) Áreas de Oportunidad Para Mejorar la IA 11(2): 66-68.
- Crobo BG Reproductive examination and evaluation of the boar in Current therapy in large animal, Theriogenology, RS Young quist (ed.), W.B, Saunders Co, Philadelphia pp. 664-669.
- De Mirjin (1997) A Inseminación Artificial en Granjas Porcinas. Memoria del 1er Curso Internacional de Producción Porcina, Mayo 5-7, Distrito Federal Mséxico (DF) Asociación de Médicos Veterinarios Especialistas en Cerdos (AMVEC) p. 15-45.
- (1999) Departamento Técnico de Minitube Mé Preparación de Diluente para Semen un Componente Crítico para una Exitosa I. A. Articulo Spermnotes The A. I. Newsletter from Minitube Of. América 111(2): 14-17.
- Falcón zjc (1992) Evaluación de la Motilidad y Daño Acrosomal de Espermatozoides de Cerdo Diluidos en BTS Utilizando Gentamicina y Neomicina Como Antibióticos, Almacenado Durante Tres Días (Tesis deLicenciatura). México (Distrito Federal). Facultad de Medicina Veterinaria y Zootecni UNAM.
- Flowers LW (1998) Main causas of Reproductive failure dueto Artificial Inseminatió Memoria del V Simposium Internacional de Reproducción e Iseminación Artificial en Porcinos p. 17-25.
- Flowers LW (2002) Manejo del verraco utilizado para inseminación artificial. Revista Aconteces Porcino 11(5): 99-103.
- Fresh Boar Semen, Swine News, (23) N°. 11; 2000, North Carolina. Cooperative Extensión Service.
- Fuentes MG, Rosales TAM (2003) Influencia de la Fracción del Eyaculado en la Relación entre Parámetros de Evaluación Espermática y del Plasma Seminal en Cerdos de Razas Comerciales. IV Jornada Internacional e Producción Porcina. mayo 22-23 Distrito Federal (México) Facultad de Medicina Veterinaria y Zootecnia UNAM, Novartis pp. 149-153.
- García RJA, Lapuente S, Corchera D, Sagues A, Martin RS (1998) Evaluación Practica del Semen. Importancia de los Resultados de Fertilidad. Memoria V Simposium Internacional de Reproducción e Inseminación Artificial en Porcinos mayo 4-6; León, (Gto) México, AMVEC pp. 27-36.
- Grandía TJ (1999) Patología en Centros de Inseminación Artificial. Revista los porcicultores y su entorno 7: 16-20.
- (1988) Gobierno del Estado de Puebla, Secretaría de Gobernación, Los Municipios de Puebla, Mexico 1st edició
- (1970-1992) Gobierno del Estado de Puebla, Consejo Estatal de Población, Síntesis Sociodemográ
- International pig topics (2003) on fram al-how to avoid getting it wrong! By Richard Openshaw, Rotech Breeding equipment. Ltd, Oving, Chichester, West Sussex Po20 2BX, UK 18(2): 11-12.
- International pig topics (2003) mahagement. By Stuar. Lumb onfarm AI –an overview. England 18(1): 11-12.
- International pig topics (2003) On Form semen collection a viable risk? By John Goss, Commercial Genomics Programme manager, pic uk, fyfield wick, abingdon, oxon OX13 5NA, UK, England 18(4): 15.
- Johnson L (1988) Current developments in swine semen: Preservation, artificial insemination and sperm sexing in the 15th IPVS Congress Birminghand England.
- Johnson L (1985) Fertility Results Using Boar Freezing Semen: 1970-1985 In Deep Freezing of Boar Semen, Uppsala Suecia pp. 199-222.
- Kenedy B, Wilkins JN (1984) Boar Breed and environnemental factors, influencing semen characteristics of boar used in Al Can. J Ani Sci 64: 833-843.
- Kubus SA (1998) Equipo técnico manual de usuario del programa informático para gestión de centros de inseminación artificial porcina: MR-A Win Pro.
- Landsverk k Their impact on fertility. IV International Conference on Boar Semen Preservation. Editado por L. A Johnston y H .D Guthrie. Maryland, EU pp. 137-139.
- Lapuente S (1995) gestión MR-A Programas informáticos para centros de inseminación artificial porcina KUBUS S. Madrid A.
- Laforest JP, Allard D (1995) Comparison of four extenders for long-term storage of fresh boar semen. In: III Conf. On boar semen preservation. Mariensee, Germany.
- Mahan D (1998) Nutritient requiremennts of the boar. Memoria del V Simposium Internacional de Reproducción e Inseminación Artificial en Porcinos; Mayo 4-6 León (Guanajuato) México; AMVEC pp. 181-192.
- Martínez, MC, Becerril AJ, Conejo NJ (1992) Motilidad y Morfología Acrosomal. En Espermatozoides de Cerdo Almacenados en los Diluyentes GEPZ Y BTS. Memoria de XXVII Congreso Nacional AMVEC, Acapulco, guerrero, México p. 91-94.
- (2000) Memorias XXXV Congreso AMVEC, Acapulco.
- Mendoza AO (2004) Consideraciones Prácticas Sobre el Manejo del Semental y su Impacto en la Productividad. Revista Acontecer Porcino 12(64): 52-63.
- Mora FJ, Laborda UL, Prieto GL (2002) Manejo de las Futuras Reproductoras. Memoria del IV Simposium del Curso de Reproducción e Inseminación Artificial Porcina (28-30), Tlaxcala, Tlax.
- Paquignon M, Mergoumn D, Courot M, DuMesnil du buisson F (1974) Technolonie de la congelación de la semence de verrat: étude in vitro. In: JRP en France 6: 71-76.
- Paquignon M, (1973) DuMesnil du buisson F. In: Fertility et prolificité destruies inseminées avec du sperme congelé. In: JRP en France p. 5.
- PIC México Visión Técnica (1999) Departamento de Producció Nuevas Alternativas ella Inseminación Artificial, La Contaminación del Semen Porcino. Artículo. Agosto 2(22): 67.
- PIC México Visión Técnical (1998) Departamento de Producción. Aspectos a Considerar por un Inseminador Altamente Efectivo. Articulo. Marzo de 2(14).
- (2000) Pork production – Manitoba agriculture and food. Al and its influence on productión efficiency. Mayo.
- Producción Porcina (1996) Distrito federal (México). Facultad de Medicina Veterinaria y Zootecnia UNAM. Elenco pp.99-117.
- Rillo M (1990) In: RIPP Problémes de reproductión lies aux anormalies de la semence de verrat. RIPP90 Loudéac France pp. 113-127.
- Roozeboom KJ, SeeT, FlowersB (2000) Manejo de la Infertilidad Estacional de los Cerdos 11: Nutrición, Hormonal y Gené Revista Cerdos (swine) 34: 28-31.
- Roozeboom KJ (2001) Factores Importantes en la Calidad del Semen Fresco. Revista Cerdos (swine) 41: 29-30.
- Signoret JP, Du Mesnil du, buisson F, Mauleon P (1972) In: Effect of mating on the onset and duration of ovulation in the sow. Journal Repro fertil 31(2): 327-330.
- Singleton WL Guía Básica para la Recolección del Semen Porcino, Evaluación y Procesamiento. Articulo departamento de ciencia animal, universidad de purdue, west lafayette.
- (1998) Spermanova can boar semen be frozen? If so, how long? Spermnotes the AI. Newsletter from Minitube of America 11(4): 10.
- Stone BA (1982) In: Haet induced infertility of boar: The interrelationship between depressed sperm out and fertility as an estimation of the critical temperature above which sperm output is impaired. Anim Repro Sci 4(4): 283-299.
- Soede NM, Kemp B (1997) In: Oestrus detection and timing of ovulation in pigs. V Int Cong, On pig reproduction The Netherlands.
- (1994) The swine AI book PIC. En presa.
- Ubeda EJL (1996) Sugerencias para lograr un centro de I, A Exitoso Revista Acontecer Porcino 11(17): 4-12.
- Wabersky D (1997) Effects of components on ovulation and fertilization. In: Journal Reprod Fert Suppl 52: 91-103.
- Weitze KF, Wagner-Rietschel H, Wabersky D (1994) In: The onset after weaning, heat duration and ovulation as major factors in Al timing in sows. Repro In demestic animals 29: 433-443.
- Wettmann RP, Bazer FW (1985) In: Influence of environmental temperature on prolificity of pigs. J Repro Fert Suppl 33: 199-208.






























