Treatment of Phenol from Aqueous Solutions by Untreated and Modified Pomegranate Peel
Torosyan GH*, Davtyan VA, Petrosyan MZ
National Polytechnic University of Armenia (NPUA), Department of General Chemistry & Chemical Technology
Submission: January 01, 2023; Published: January 19, 2023
*Corresponding author: Torosyan GH, National Polytechnic University of Armenia (NPUA), Department of General Chemistry & Chemical Technology
How to cite this article: Torosyan GH, Davtyan VA, Petrosyan Z. Treatment of Phenol from Aqueous Solutions by Untreated and Modified Pomegranate Peel. Ann Rev Resear. 2023; 8(2): 555735. DOI: 10.19080/ARR.2023.08.555735
Abstract
The aim of this investigation was to determine the ordinary and modified analog of pomegranate peel as good adsorbent for phenol removal from water solution. The modified analog shows higher adsorptive activity. The Langmuir adsorption model was used for description of the adsorption processes and for mathematical decision of adsorption theoretical & experimental results. Butch adsorption studies based on experimental kinetic results obtained. The obtained results indicate the efficiency of pomegranate peels as low-cost adsorbent for phenol removal from aqueous solution. It has been shown that adsorption treatment of wastewater from phenol with proposed adsorbents can be a classic example of the reductive treatment of phenol for further use. Adsorption activity of obtained adsorbents was assessed using infrared spectroscopy measurements. Equilibrium isotherms were analyzed using the Langmuir isotherm equation, which describes the equilibrium state of the adsorption process. The equilibrium data fits the Langmuir isotherm.
Keywords: Pomegranate; Raw and Modified Peel; Acid Treatment; Phosphoric Acid; Adsorption; Equilibrium Isotherms; Langmuir Isotherm
Introduction
Industrial effluents treatment from dissolved in water organic substances the most important and at the same time one of the most difficult to solve. For example, phenol, which is widely used, and therefore ends up in aqueous media, is especially dangerous due to its relatively good solubility in water. Phenol is hardly biodegradable, it has high toxicity, and it is highly mobile in ecosystems [1]. It is known that aqueous solutions of phenol are widely used as model systems in the study of industrial wastewater treatment processes due to the large volumes of its production (for example, Global phenol demand amounted to about 12 million metric tons in 2022 [2]) and, therefore, its wide prevalence as pollutant. The release of phenol into the hydrosphere leads to an undesirable water odor [1] and a decrease in sunlight penetration, and some phenol derivatives are also toxic / carcinogenic /. Methods for water treatment from phenol dissolved in water are divided into two groups - destructive / decomposable / and regenerative / restorative /.
In this article, adsorption treatment of wastewater from phenol is given as a classic example of the reductive treatment of phenol for further use. Recently, comparative studies have been reported on the adsorption of phenol between agricultural solid waste and some other inexpensive adsorbents [3]. Despite the introduction into practice of more and more new adsorbents, activated carbons, being perhaps the oldest industrial sorbents, continue to be the most common materials used for cleaning from a wide variety of contaminants, as target products of chemical, pharmaceutical, food and other industries, and their wastewater. Such a long and widespread use of activated carbons as sorbents is explained both by the diversity of the porous structure, which varies depending on the origin of the coal and the conditions for its activation, and the availability of the feedstock. Practically, any carbon-containing material can be processed into activated carbon [3]. Activated carbons are offered from fruit seeds, from grains of wheat and corn, corn cobs, beans, sunflower husks, pecan shells, hazelnuts and coconuts, almonds, etc. [4].
Physical modification includes appropriate sizing of adsorbents by milling and grinding, heat treatment (steam and microwave), ultrasonic irradiation, mixing, freezing, drying, and high pressure (autoclaving). The right choice of adsorbent modification additives is critical to achieving greater efficiency and more usability cycles. Chemical modification involves the treatment of adsorbents with a variety of chemicals, which can be mineral acids (hydrochloric acid, sulfuric acid, nitric acid, phosphoric acid), organic acids (oxalic acid, citric acid, tartaric acid, salicylic acid), chelating agents, etc. [5]. In the present study, acid modification was carried out with phosphoric acid. The pomegranate is one of the most popular fruits in the world due to its pleasant taste, high nutritional value, as well as many medicinal properties. Pomegranate is widely used in the fruit juice industry, especially pure pomegranate juice. The fruit consists of the edible part, seeds, and peel. Pomegranate peel makes up from 5% to 15% of its total mass [6].
Materials and Methods
The preparation of sorbents from pomegranate peel. The pomegranate peel was initially washed with distilled water and dried in sunlight until the moisture had partially evaporated. Complete evaporation was followed by placing the pomegranate in an oven at 70ºC for 2-3 days to remove moisture before use. Dried pomegranate peels were ground in a ball mill. Then part of the dried and crushed peel was kept for 24 hours in a solution of phosphoric acid (30 wt.%, ratio 1: 1). The samples were washed several times with double distilled water to remove excess acid and then dried in an oven at 100°C. The analysis of the surface of the modified samples was previously carried out int the laboratory sorbents of the Institute of general & inorganic chemistry NAS RA [7]. Treatment of pomegranate peel with phosphoric acid increased the specific surface area, which ranged from 105,0 m2/g to 240,0 m2/g respectively. Phosphoric acid is the preferred modifying agent among mineral & organic acids to improve the structural integrity of the molecular sieve backbone [8].
The obtained particles of the modified sorbent were studied by infrared spectroscopy to find active sorption centers. IR spectroscopy was carried out on a Specord-75 IR spectrophotometer in Vaseline oil (1.5 mg of sorbent per 0.5 mg of Vaseline) in air [9]. The spectra were measured from 3700 to 700 cm-1. Peaks at 3430 cm-1 show O-H stretching vibrations and spectrum bands. The peaks observed at 2920 cm-1 are C-H stretching vibrations of aliphatic C-H. The peaks at 1230 cm-1 and 1030 cm-1 correspond to the O-H bending vibration and the C-O extension vibration of the carboxylic acid. The carbon spectrum obtained by activation with phosphoric acid, the band in the region of 1300-900 cm-1 is caused by phosphorus-oxy-containing functional groups. The studies for adsorption activities. It was carried out under static conditions on a laboratory rocking chair. It is known that static single-stage adsorption is used in cases where the adsorbent is very cheap or is a waste product. Sorbents in an amount of 1.0 ± 0.01 g were added to aqueous solutions with a volume of 100 ± 0.1 ml containing phenol (in an amount from the maximum solubility to the MPC for the substance). Next, the mixture was placed on a shaker and subjected to stirring for 6 hours at a temperature of 20°C, then the sample was settled for 24 hours. Residual amounts of phenol were determined by UV spectrophotometry and HPLC. filled with micro spherical silica gel sorbents with C18 groups on the surface, mobile phase flow rate 1 ml/min. UV-254 nm detector.
Results and Discussion
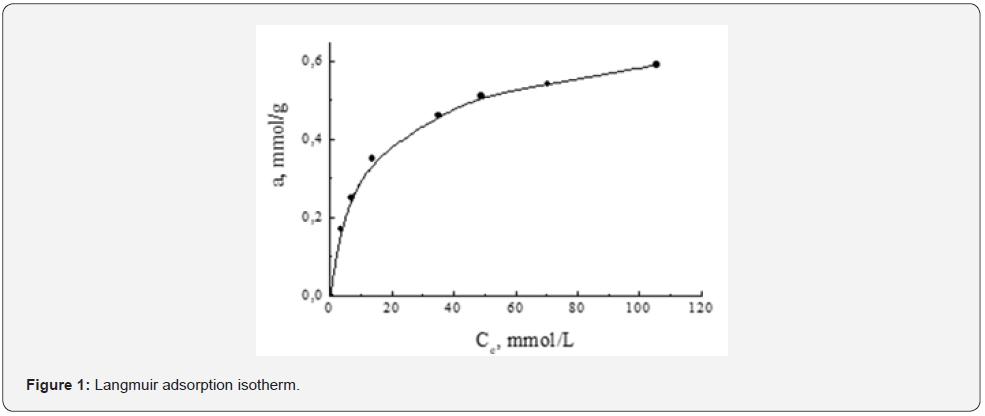
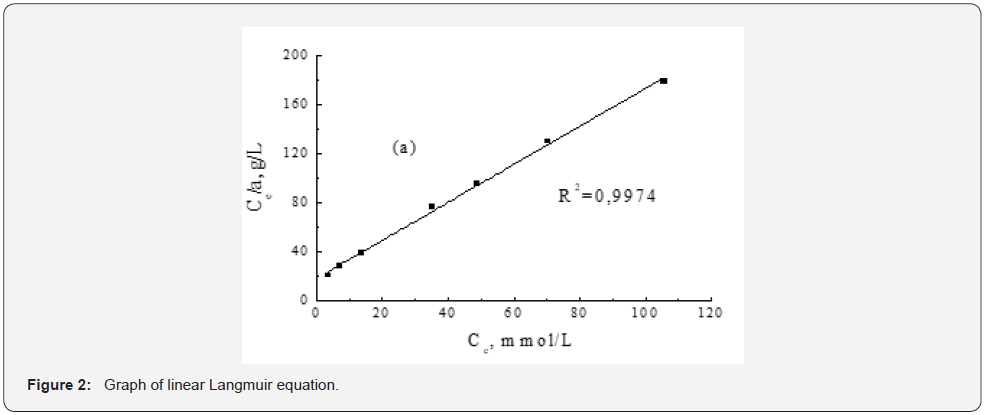
Kinetic studies were carried out for a modified analogue of pomegranate peel due to the higher purity of the object under study. To determine the adsorption characteristics in relation to phenol, the most appropriate model was chosen to describe the adsorption equilibrium (Table 1). The results were simulated using simple Langmuir adsorption isotherms, which previously showed the efficiency of phenol adsorption in the studied concentration range and temperature of 20 ºC [10]. The optimal conditions for its removal of phenol from the solution were determined. With these optimal parameters, the adsorption isotherm shown in Figure 1 was obtained. As can be seen from Figure 1, the shape of the isotherm corresponds to the Langmuir type [9]. The initial section at low phenol concentrations (up to 6.0 mmol/l) has a steep slope and is almost straight. The second isothermal section (concentration up to 105 mmol/l) is characterized by a decrease in the adsorption rate of phenol with an increase in its concentration in solution. This, apparently, is associated with an increase in the population density of phenol molecules on the surface of the sorbent and an increase in the electrostatic repulsion between them. However, saturation of the sorbent with phenol was not observed. Figure 2 & 3 shows the linear shapes and the correspondence of the Langmuir model to the experimental data. Graphs of linear Langmuir equation (Figure 2) for experimental data on phenol adsorption show that they fit well on straight lines, which confirms the fact that this model can be used to describe phenol sorption. As can be seen from Figure 3, this model correlates very well with adsorption data.

Conclusion
It has been shown that untreated and modified (treated with phosphoric acid) pomegranate peels show adsorption activity when removing phenol from an aqueous solution. A comparative study showed that the sample treated with phosphoric acid showed a higher adsorption capacity. The adsorption activity of obtained adsorbents can assessed using infrared spectroscopy method. Equilibrium adsorption is practically reached in 65 min. Equilibrium data are interpreted by the Langmuir isotherm model than by other known theoretical models. Adsorptive treatment of wastewater from phenol with ordinary and treated by phosphoric acid then modified analog can be a classic example of the reductive treatment of phenol for further use.
References
- Abdollahi M, Hassani S, Derakhshani M (2014) The Encyclopedia of Toxicology (2014), The third edition, Academic Press is an imprint of Elsevier pp. 871-873.
- (2022) Phenol in Chemical Economics Handbook (CEN), S&P Global, UN New-York.
- Naga S, Bhardwaja A, Pandeya P, Arorab M, Babu JN (2018) Use of Agricultural Solid Wastes as Adsorbents, Published online p. 35.
- Mohamed Salleh MA, Mahmoud DK, Abdul Karim WW, Idris A (2011) Cationic and Anionic dye adsorption by Agricultural Solid Wastes: A comprehensive review, Desalination 280(1-3): 1-13.
- Patel S (2012) Potential of fruit and vegetable wastes as novel bio sorbents: summarizing the recent studies, Environ. Sci. Biotechnol 11: 365-380.
- Moghadam MR, Nasirizadeh N, Dashti Z, Babanezhad E (2013) Removal of Fe (II) from aqueous solution using pomegranate peel carbon: equilibrium and kinetic studies, International Journal of Industrial Chemistry 4: 19-26.
- Girgis BS, Attia AA, Fathy NA (2007) Modification in adsorption characteristics of activated carbon produced by H3PO4 under flowing gases, Colloid Surface A. Physicochem Eng. Aspect 299(1-3): 79-87.
- Brown D, Floyd A, Seinzberi M (1992) Spektroscopy of organic substances М: Мir pp. 385.
- Tóth J (2000) Calculations of the BET-Compatible Surface Area from any type I Isotherms Measured above critical temperature, Journal of Colloid and Interface Science 225(2): 378-383.






























