Preparation of a DNA (E. coli) Crown Cell line in Vitro-Microscopic Appearance of Cells
Shoshi Inooka*
Japan Association of Science Specialists, Japan
Submission: November 23, 2022; Published: December 06, 2022
*Corresponding author: Shoshi Inooka, Japan Association of Science Specialists, Japan
How to cite this article: Shoshi I. Preparation of a DNA (E. coli) Crown Cell line in Vitro-Microscopic Appearance of Cells. Ann Rev Resear. 2022; 8(1): 555728. DOI: 10.19080/ARR.2022.08.555728
Abstract
DNA crown cells (artificial cells), in which the outside of the membrane is covered with DNA, can be readily synthesized in vitro using sphingosine (Sph)-DNA-adenosine mixtures. These DNA crown cells can proliferate within egg whites. A previous report described how assemblies of such synthetic DNA crown cells were formed and how they changed into crystal-like substances after the addition of monolaurin. Further monolaurin addition increased the number of cells. The aim of this study was to clarify whether such cells could be cultivated over 14 days. The findings showed that cells could be cultivated for approximately 2 months through seven generations. Cells cultivated for a long time qualify as a cell strain. Here, the preparation of such a strain is described in conjunction with microscopic observations.
Keywords: Synthetic DNA crown cells; Cell strain; Sphingosine-DNA; Cell culture
Introduction
Artificial cells are cells covered with DNA and referred to as DNA crown cells [1-3]. Synthetic DNA crown cells can be prepared with sphingosine (Sph)-DNA and adenosine-monolaurin (A-M) compounds, and DNA crown cells can be generated by incubating synthetic DNA crown cells in egg white. In a previous study [4], assemblies of synthetic DNA (E. coli) crown cells were produced and converted into crystal-like substances with monolaurin addition. Moreover, these assemblies of cells proliferated with further stimulation with monolaurin, forming crystal-like substances [5]) that could be cultivated for 14 days using egg white as a culture medium [6,7]. The present study examined whether such cells could be cultivated more than 14 days. The findings showed that such cells could be cultivated over 50 days through seven generations and these cells qualified as a strain. Here, the preparation of such a strain is described in conjunction with microscopic observations.
Materials and Methods
Materials
The following materials were used: Sph (Tokyo Kasei, Japan); DNA (E. coli B1 strain, Sigma-Aldrich, USA); adenosine (Sigma-Aldrich; Wako, Japan); and monolaurin (Tokyo Kasei), A-M; a compound synthesized from a mixture of adenosine and monolaurin) [8]. Monolaurin solutions were prepared to a concentration of 0.1M in distilled water. Eggs was obtained from a local market and egg white was collected from the egg. In culture, Dulbecco’s minimal essential medium (D-MEM) containing 10% bovine serum (Sigma-Aldrich) was used.
Methods
Preparation of DNA crown cells: Synthetic DNA crown cells was prepared as described previously [8,9]. Briefly, l80 μg of Sph (10 mM) and 90 μL of DNA (1.7μg/μL) were combined, and the mixture was heated twice. A-M solution (100 μL) was added, and the mixture was then incubated at 37°C for 15 min. Next, 30 μL of monolaurin solution was added and the mixture was incubated at 37°C for another 5 min. The resulting suspension was used as the synthetic DNA (E. coli) crown cells.
Preparation of samples in culture: A total of 25 μL of synthetic DNA crown cells was incubated for 18 h at 37°C followed by the addition of 25 μL of monolaurin and incubation for 18 h at 37ºC. Then, 25 μL of monolaurin was added to the mixtures and incubated for 15 min at 37°C.
Cell culture procedures: A total of 20 μL of sample was added to 200 μL of egg white and incubated for 7 days at 37°C (primary culture). Then, 20 μL of sample was added to 200 μL of fresh egg white and incubated for 7 days at 37°C (secondary culture). Further, 20 μL of sample was added to 200 μL of fresh egg white and incubated for 7 days at 37°C (third culture).
To scale up the first culture, 0.1 mL of sample was added to 1.0 mL of fresh egg white and incubated for 7 days at 37°C (primary scale-up cultures; fourth culture). The second scale-up culture was produced by adding 0.1 mL of sample from the primary scale-up culture was added to 1.0 mL of D-MEM and incubated for 10 days at 37°C (second scale-up culture; fifth culture). As the third scaleup culture, 1.0 mL of sample from the secondary scale-up culture was added to 10.0 mL fresh D-MEM and incubated for 10 days at 37°C (sixth culture). Moreover, in the next generation, 1.0 mL of the sample from the third scale-up culture was added to 10.0 mL of D-MEM and incubated for 10 days at 37°C (fourth scale-up culture, seventh culture). After incubation, the culture medium was stored at approximately 4°C. For observations, approximately 9.5 mL of culture medium in a sample was removed leaving approximately 0.5 mL of culture medium, which was used as the final sample in microscopic observation.
Microscopic observations: A total of 20 μL of sample was placed on a slide glass and covered with a cover glass. The slides were then observed under a light microscope.
Results and Discussion
Microscopic appearance of synthetic DNA (E. coli) crown cells in final sample
The microscopic appearance of synthetic DNA crown cells in primary and secondary cultures was reported previously. Here, the microscopic appearance of synthetic DNA crown cells in final cultures is presented. Cells stored at appropriate 4°C and naturally precipitated to the bottom of the tubes in which they were stored. Approximately 9.5 mL of the upper fluid was aspirated and the cells at the bottom were suspended in 0.5 mL of the culture medium. Then, 20 μL of the solution was placed on a glass slide, covered with a cover glass, and observed under a microscope. Figure 1 shows numerous cells and cell clusters (arrows a, b, c, d, e, f, and g). Cells measured approximately 3–5 μm (arrow g). Figure 2 shows a magnification of the structure shown by arrow a in Figure 1. The colors of the structures facilitate observations however, the different colors indicate different substances. Thus, for convenience, yellow-green structures are called o-structures, and orange structures are called m-structures; most objects consisted of o-structures (Figure 2 arrow a) and m-structures (Figure 2 arrow b). A typical cell was observed (Figure 2 arrow c). A cell derived from an object (Figure 2 arrow a) was observed (Figure 2 arrow d). An object that may have fused was observed (Figure 2 arrow e).
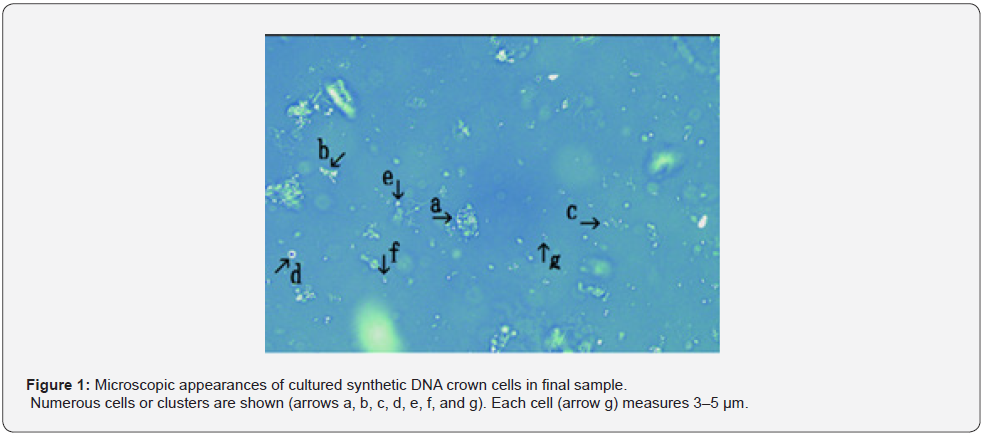
Figure 3 shows a magnification of the structure shown by arrow b in Figure 1. All objects consisted of o-structures (Figure 3 arrow a), which enclosed m-structures (Figure 3 arrow b). A cell derived from the structure indicated by arrow b in Figure 3 was observed (Figure 3 arrow c). Two objects were connected o-structures (Figure 3 arrow d). An object capable of division was observed (Figure 3 arrow e). Figure 4 showed magnified picture in Figure 1 arrow c. A object which consists of o-structure (Figure 4 arrow a) and m-structure (Figure 4 arrow b) was observed.
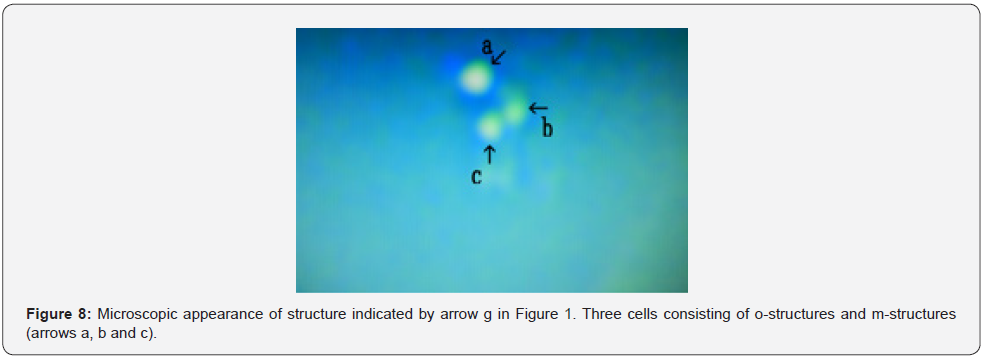
Figure 5 shows a magnification of the structure shown by arrow d in Figure 1. A typical cell consisting of o-structures on the outside (Figure 5 arrow a), m-structures in the middle (Figure 5 arrow b), and possibly o-structures in the inside (Figure 5 arrow c) was observed. The inside of the cell was empty (Figure 5 arrow d). Figure 6 shows a magnification of the structure shown by arrow e in Figure 1. A cell-like object consisting of o-structures (Figure 6 arrow a) and m-structures (Figure 6 arrow b) was observed. Figure 7 shows a magnification of the structure shown by arrow f in Figure 1. A object consisting of o-structures (Figure 7 arrow c) and m-structures (Figure 7 arrow b) was observed. The o-structure appeared to develop from a part of the o-structure (Figure 7 arrows a and c). Figure 8 shows a magnification of the structure shown by arrow g in Figure 1. Three cells consisting of o-structures and m-structures were observed (Figure 8 arrows a, b and c).
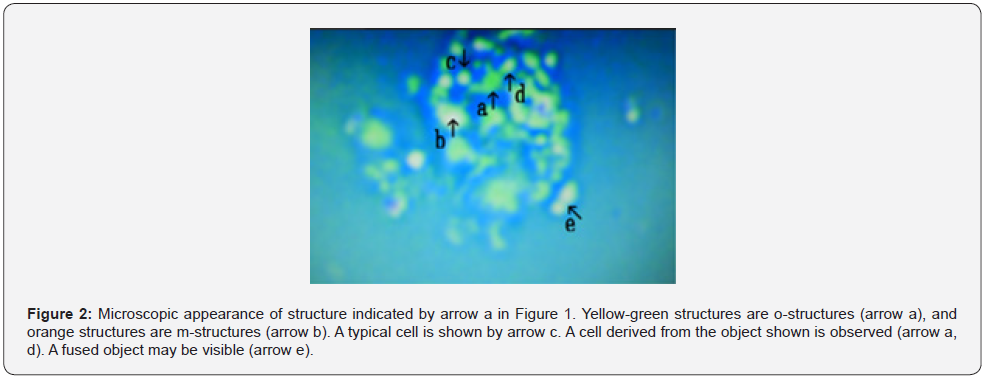
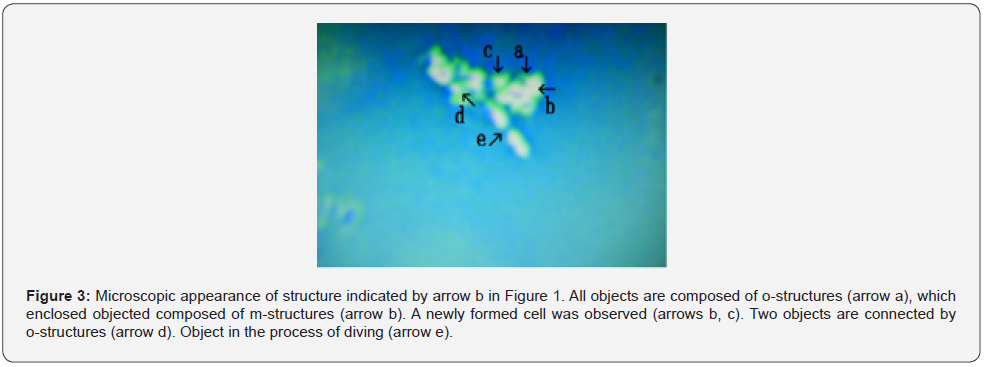

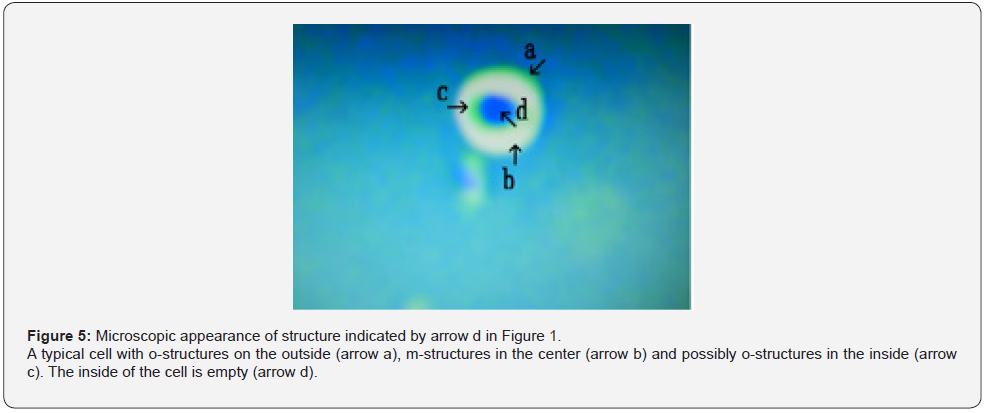


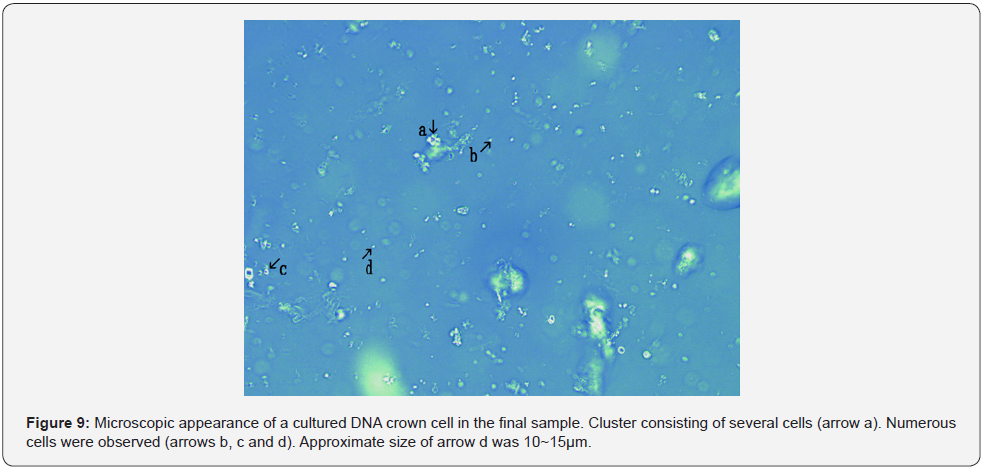
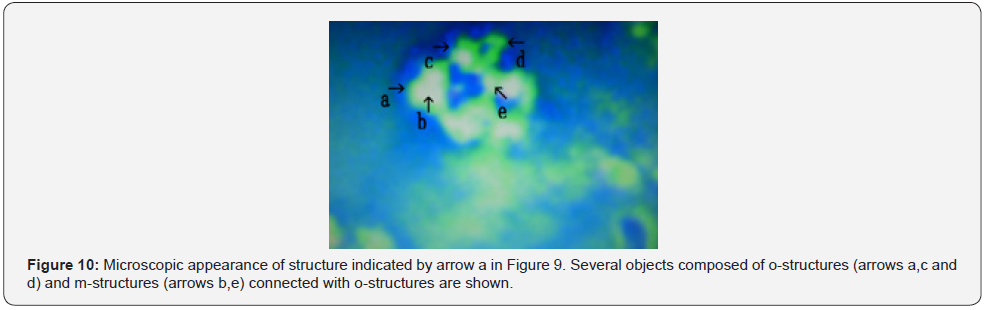
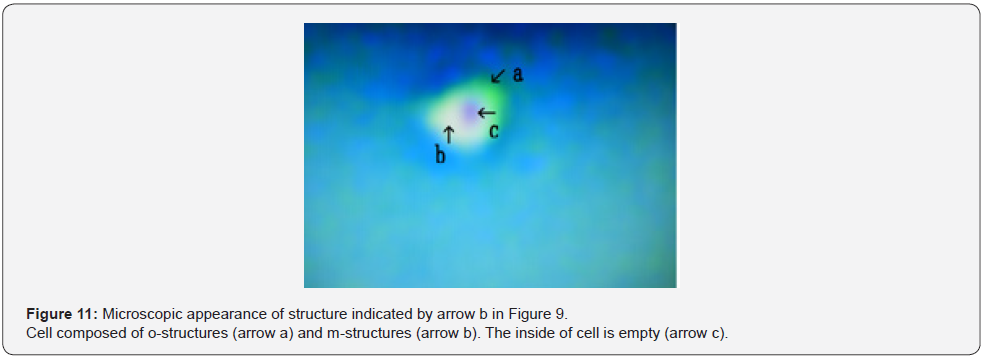
Figure 9 shows microscopic appearance of a cultured DNA crown cells in the final sample. Cluster consisting of several cells (Figure 9 arrow a). Numerous cells were observed (Figure 9 arrows b, c and d). Approximate size arrow d was 10~15 μm. Figure 10 shows a magnification of the structure shown by arrow a in Figure 9. Several objects comprised of o-structures (Figure 10 arrows a, c and d) and m-structures (Figure 10 arrows b and e) were observed; these structures were connected by o-structures. Figure 11 shows a magnification of the structure shown by arrow b in Figure 11. A cell consisting of o-structures (Figure 11 arrow a) and m-structures (Figure 11 arrow b) was observed. It is not clear whether the cell contained another substance (Figure 11 arrow c).
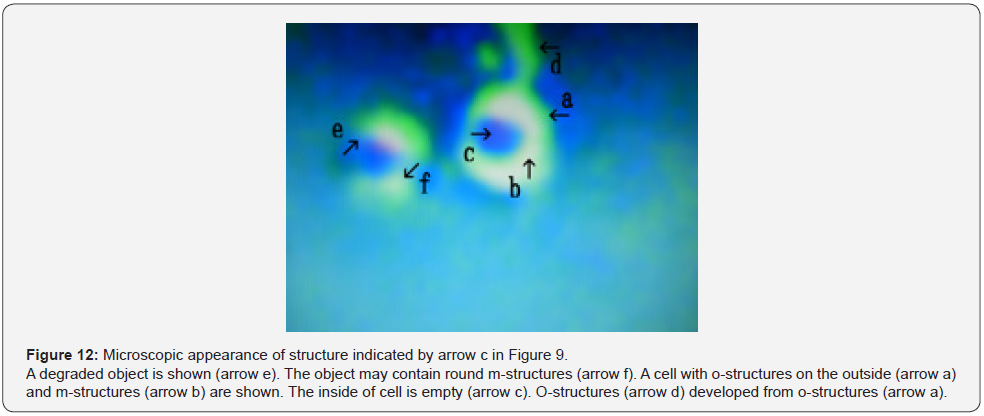
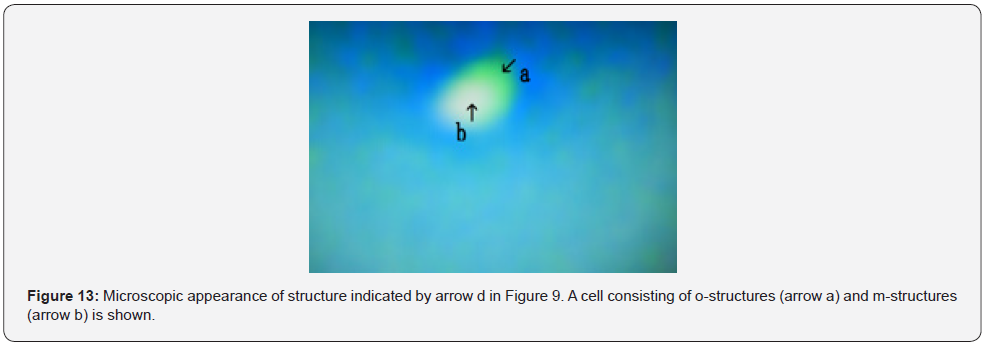
Figure 12 shows a magnification of the structure shown by arrow c in Figure 9. A degraded object, possibly composed of m-structures, was observed (Figure 12 arrow e). The object was round (Figure 12 arrow f). In addition, a cell covered by o-structures (Figure 12 arrow a) and m-structures (Figure 12 arrow b) was observed. The inside of cell was empty (Figure 12 arrow c). O-structures (Figure 12 arrow d) develop from o-structures (Figure 12 arrow a). Figure 13 shows a magnification of the structure shown by arrow d in Figure 9. A cell consisting of o-structures (Figure 13 arrow a) and m-structures (Figure 12 arrow b) was observed.
Synthetic DNA crown cells were cultured for seven generations over approximately 50 days and many cells and related objects were observed in the culture medium. The findings showed that synthetic DNA cells could be cultured for extended period. On the other hand, it was not clear whether the cells or related objects that were observed in the present experiments were present in the original samples. Samples of cultures (20 μL) were used to start the cultures, and samples were cultivated for seven generations.
If the cells in the original sample contained 106/mL and did not grow, no cells were observed after the seventh generation, Also, in a previous study [5], it was demonstrated that cells proliferate from assemblies that were formed with synthetic DNA crown cells after the addition of monolaurin.
Therefore, the aim of the culture study was to clarify whether the cells that were observed to reproduce in the monolaurin treatment [5] were alive or not. However, the aim of the study changed to determining whether the synthetic DNA crown cells could be cultivated. In previous studies [6,7], such cells could be cultivated, and, in the present paper, it was demonstrated that a strain of such cells could be obtained. On the other hand, it took a long time to obtain such a strain because there was a question about whether the materials, including cells or materials of the original samples, were retained in the medium after cell culture. To clarify this question, cultures were performed for seven generations, and it was demonstrated that cells which were cultivated were not associated with the materials in the original samples. It may therefore be possible to establish a strain of synthetic DNA crown cells in a more convenient manner, such as by decreasing culture time, or using cell culture medium instead of egg white. On the other hand, the microscopic appearance of the cultured cells showed that they consist of o-structures (yellow green) and m-structures (orange). Because the outside of DNA crown cells consists of sphingosine-DNA, it was clear that o-structures were constructed from sphingosine-DNA-related components.
O-structures often elongated and formed a ring. This phenomenon may be indicative of the mechanisms of cell formation; for example, sphingosine-DNA-related components grew (became elongated) and formed cells. Importantly, in this study, synthetic DNA crown cells were successfully cultivated in D-MEM, implying that non-organisms developed into organisms. To clarify the technique, numerous strains of synthetic DNA crown cells need to be developed in future. Therefore, future studies will clarify the methods required to optimize the establishment of synthetic DNA crown cell strains as well as DNA (E. coli) crown cell strains.
References
- Inooka S (2012) Preparation and cultivation of artificial cells. App Cell Biol 25: 13-18.
- Inooka S (2016) Preparation of Artificial Cells Using Eggs with Sphingosine-DNA. J Chem Eng Process Technol l7(1): 277.
- Inooka S (2016) Aggregation of sphingosine-DNA and cell construction using components from egg white. Integrative Molecular Medicine 3(6): 1-5.
- Inooka S (2022) The assembly in synthetic DNA crown cells with inorganic salts and the transformation of DNA crown cells to crystal like substance in the presence of monolaurin. Novel Research in Science 11(2): NRS.000759.
- Inooka S (2022) Cell proliferation from the Assembly of Synthetic DNA (E. coli) Crown Cells with Stimulation by Monolaurin Twice. Current Trends on Biotechnology & Microbiology 2(5): 2022.
- Inooka S (2022) Microscopic appearance of synthetic DNA (E. coli) crown cells in primary culture. Applied Cell Biology Japan 35: 71-98.
- Inooka S (2022) Microscopic Appearance of Synthetic DNA (E. coli) Crown Cells in Secondary Cultures, Novel Research in Science 12(4): NRS 000791.
- Inooka S (2017) Systematic Preparation of Artificial Cells (DNA Crown Cells) J Chem Eng Process Technol 8: 327.
- Inooka S (2017) Biotechnical and Systematic Preparation of Artifical Cells. The Global Journal of Research in Engineering 17(C1): 1-10.






























