Dependence on Various Factors of Ribulose Bisphosphate Carboxylase/Oxygenase Activity in Extracts from the Leaves of Arabidopsis of the Enkheim Race
Mirzorakhimov AK1*, Babadzhanova MA2 and Saifuddinov AK3
1Vice-rector for Science of the Tajik State Pedagogical University named after Sadriddin Aini, Tajikistan
2Аssociate Professor, Department of Plant Physiology and Biotechnology, Tajikistan National University, Tajikistan
3Professor, Department of Plant Physiology and Biotechnology, Tajikistan National University, Tajikistan
Submission: March 03, 2022; Published: March 21, 2022
*Corresponding author: Mirzorakhimov AK, Vice-rector for Science of the Tajik State Pedagogical University named after Sadriddin Aini, Tajikistan
How to cite this article: Mirzorakhimov A, Babadzhanova M, Saifuddinov A. Dependence on Various Factors of Ribulose Bisphosphate Carboxylase/ 002 Oxygenase Activity in Extracts from the Leaves of Arabidopsis of the Enkheim Race. Ann Rev Resear. 2022; 7(2): 555710. DOI: 10.19080/ARR.2022.07.555710
Abstract
The obtained results indicate that optimal conditions of the reaction medium have been selected for the manifestation of the carboxylase activity of ribulose bisphosphate carboxylase/oxygenate of the multi-enzyme complex of the Calvin cycle in extracts from the leaves of arabidopsis of the Enkheim race. When the carboxylase activity of ribulose bisphosphate carboxylase/oxygenase of the multiferment complex of the Calvin cycle is manifested, a positive cooperative interaction between the active centers of the enzyme subunits is characteristic.
Abbreviations: SSVS: Superior supervisor support; NMEP: National Malaria Elimination Program; IRS: Indoor Residual Spraying; DDT: Dichlorodiphenyltrichloroethan; LICs: low-income countries; SOPs: spray operators
Introduction
Ribulose bisphosphate carboxylase/oxygenase (RuBisCO/O 4.1.1.39) is the most abundant and important enzyme on Earth. This enzyme catalyzes the key reaction of the dark stage of photosynthesis - the addition of carbon dioxide to ribulose-1,5-phosphate, resulting in the formation of the primary organic substance -3-phosphoglyceric acid. Rubisco/O is the main enzyme in plant leaves, where its content in the stroma reaches up to 70% of the total soluble protein and up to 50% of all proteins in leaf tissues. Steel of high content of Rubisco/O reflects the important role of this enzyme. The reaction catalyzed by Rubisco/O determines the overall rate of CO2 inclusion into photosynthetic metabolism. Therefore, RuBisCo/O is assigned the role of a regulator of the intensity of photosynthesis, and photosynthesis y plays a decisive role in the production process. In this regard, it is obvious that it is necessary to deeply study the properties of RuBisCO/O in order to understand the mechanisms of regulation of the intensity of photosynthesis. RuBisCO forms a multienzyme complex with enzymes that regenerate its substrate, ribose phosphate isomerase and phosphoribulokinase [1-3], this is evidenced by the fixation of 14CO2 in the presence of ribose-5-phosphate and ATP by extracts from plant leaves [4], but the kinetic properties of the enzyme have not been studied. The aim of this work is studying the kinetic behavior of ribulose bisphosphate carboxylase/oxygenase in the Calvin cycle multienzyme complex in Arabidopsis leaf extracts.
Material and Methods
The leaves of Arabidopsis Arabidopsis Thaliana L. of the Cruciferae family of the Enheim race were taken as objects for our experimental studies. The ease of cultivation of Arabidopsis plants in soil and artificial nutrient media makes them a very convenient object for genetic and physiological-biochemical studies. Plants have a short growing season and produce abundant seed material - from 10,000 to 40,000 per plant, a small number of chromosomes - five linkage groups, a relatively small genome - 24,000 genes. Arabidopsis was grown in boxes, in a greenhouse under optimal conditions - temperature 20-25o C, soil (humus and sand in a ratio of 1:2), relative humidity 70-80%, illumination 20-25 thousand. lux. The preparation of an extract from Arabidopsis leaves is described in detail in [5]. The quantitative protein content was determined with Benedict’s reagent [6]. The calibration curve is based on bovine serum albumin. Determination of the carboxylase activity of ribulose bisphosphate carboxylase/oxygenase was carried out spectrophotometrically according to the method of Rekker, modified by A.K. Romanova [7].
Results and Discussion
To study the kinetic behavior of ribulose bisphosphate carboxylase/oxygenase in the multienzyme complex of the Calvin cycle, we studied the effect of reaction duration, protein amount, ribose-5-phosphate concentration, and carbon dioxide in the reaction medium on the carboxylase activity of the enzyme. The carboxylase activity of ribulose-1,5-bisphosphate carboxylase/ oxygenase of the multienzyme complex was determined after incubation of all components of the reaction medium for 1, 2, 4, 6, 8, 12, and 16 minutes. Table 1 shows the results of a study of the dependence on the duration of the reaction of the rate of the carboxylase reaction catalyzed by ribulose-1,5-bisphosphate carboxylase/oxygenase in extracts from the leaves of Arabidopsis of the Enkheim race. From the data presented in Table 1, it can be seen that the reaction rate decreased after one minute. Consequently, the components of the reaction medium were in the optimal ratio within 0.5-1 minute. The reaction rate was not linear; the amount of product formed in one minute did not double in 2 minutes, triple in 3 minutes, etc. Based on the data obtained, the study of the influence of various factors on the rate of the carboxylase reaction of ribulose-1,5-bisphosphate carboxylase/ oxygenase of the multienzyme complex was carried out for 1 minute.
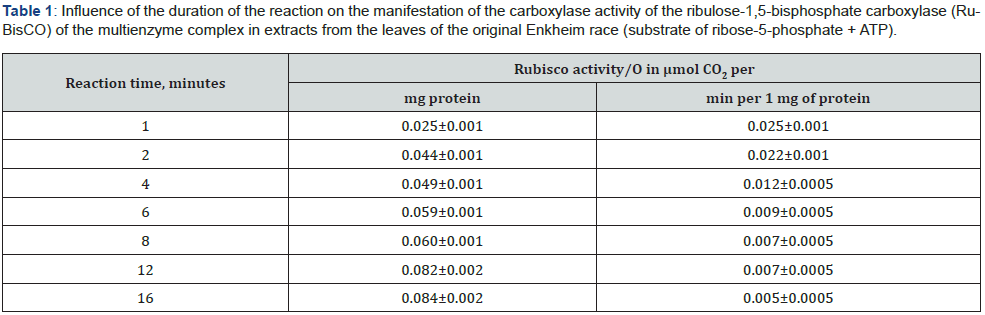
The influence of the amount of protein on the manifestation of the carboxylase activity of ribulose bisphosphate carboxylase/ oxygenase of the multiferent complex in extracts from the leaves of Arabidopsis of the Enkheim race was studied by us at a content of 10 μmol of ribose-5-phosphate, 10 μmol of ATP per ml of the reaction medium and a reaction time of 1 minute. The results of studying the dependence of the carboxylase activity of ribulose- 1,5-bis-phosphate carboxylase on the protein concentration in the reaction medium are shown in Figure 1. The influence of the amount of protein on the manifestation of the carboxylase activity of ribulose bisphosphate carboxylase/oxygenase of the multiferent complex in extracts from the leaves of Arabidopsis of the Enkheim race was studied by us at a content of 10 μmol of ribose-5-phosphate, 10 μmol of ATP per ml of the reaction medium and a reaction time of 1 minute. The results of studying the dependence of the carboxylase activity of ribulose-1,5-bisphosphate carboxylase on the protein concentration in the reaction medium are shown in Figure 1.
As can be seen from the data shown in Figure 1, the curve of the dependence of the carboxylase activity of ribulose bisphosphate carboxylase/oxygenase on the amount of protein in the reaction medium did not have a hyperbolic shape, but was a classical sigmoid shape. Within the amount of protein per ml of the reaction medium of 1–2 μg, cooperative effects of the interaction of enzyme subunits were observed, indicated by the addition of substrate molecules. With an increase in the amount of protein in the reaction medium to 5–10 μg, the carboxylase activity of ribulose bisphosphate carboxylase/oxygenase increased. The maximum reaction rate was achieved when the amount of protein per ml of the reaction medium was 10 μg. A further increase in the amount of protein per ml of the reaction medium led to a sharp decrease in the rate of the carboxylase reaction. Therefore, under these conditions of the reaction medium, the optimal amount of protein for the manifestation of the carboxylase activity of ribulose bisphosphate carboxylase/oxygenase of the multienzyme complex was 10 μg. In further studies, 10 μg of protein per ml of reaction medium was used.
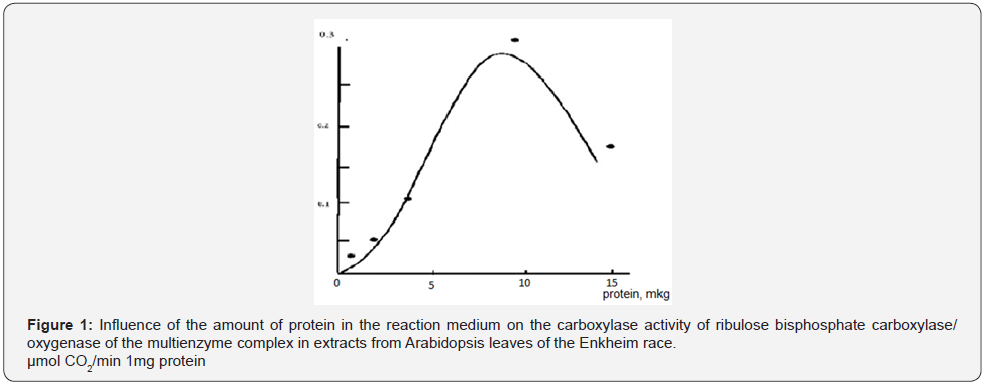
The results of studying the dependence of the carboxylase activity of ribulose bisphosphate carboxylase/oxygenase of the multienzyme complex on the concentration of carbon dioxide in the reaction medium in extracts from Arabidopsis leaves of the Enkheim race are shown in Figure 2. From the data presented in Figure 2, it can be seen that the curve of dependence on the concentration of carbon dioxide in the reaction medium of the carboxylase activity of ribulose bisphosphate carboxylase/ oxygenase had a classical sigmoid shape. This indicates complex cooperative effects in the interaction of subunits with each other, which are indicated by carbon dioxide molecules upon binding to the enzyme. The maximum carboxylase activity of ribulose bisphosphate carboxylase/oxygenase was manifested at a content of 40–50 μmol of carbon dioxide per ml of the reaction medium. Therefore, this concentration of carbon dioxide was optimal under the given conditions of the reaction medium, i.e. the enzyme is completely saturated with the substrate. In further studies, 50 μmol of carbon dioxide per 1 ml of the reaction medium was used.
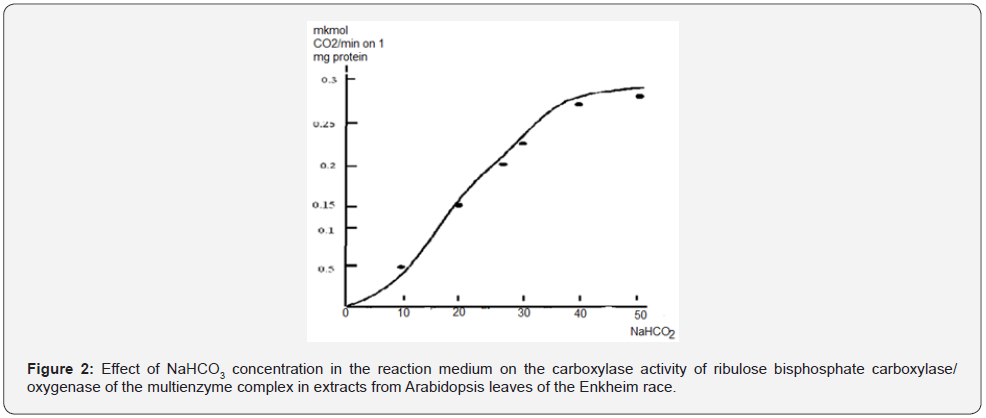
The effect of the concentration of ribose-5-phosphate on the carboxylase activity of the ribulose bisphosphate carboxylase/ oxygenase of the multienzyme complex in extracts from the leaves of Arabidopsis of the Enkheim race was studied by us at a content of 10 μg of protein, 10 μmol of ATP per ml of the reaction medium and a reaction time of 1 minute. The results obtained are shown in Figure 3.
As seen in Figure 3, the curve of dependence on the concentration of ribose-5-phosphate of the carboxylase activity of ribulose bisphosphate carboxylase/oxygenase had a complex sigmoid shape with a lag period at a content of 0.1-0.3 mm of the substrate in the reaction medium. The lag period is indicative of substrate-induced cooperative interactions between the active centers of the enzyme subunits. With an increase in the content of ribose-5-phosphate in the reaction medium to 0.5–1 mM, an increase in the carboxylase activity of the enzyme began. A further increase in the content of the substrate to 1.5 mm resulted in the maximum increase in the rate of the carboxylase reaction. Within the range of ribose-5-phosphate concentrations of 1.5–2.0 mM, the carboxylase activity of ribulose bisphosphate carboxylase/oxygenase decreased. The analysis of the obtained results showed that the optimal concentration of ribose-5- phosphate under the given conditions of the reaction medium was 1 mM. This concentration of ribose-5-phosphate was used in further studies. Thus, the optimal conditions of the reaction medium were selected for the manifestation of the carboxylase activity of the ribulose bisphosphate carboxylase/oxygenase of the multienzyme complex of the Calvin cycle in extracts from the leaves of Arabidopsis of the Enkheim race. With the manifestation of carboxylase activity of ribulose bisphosphate carboxylase/ oxygenase of the multienzyme complex of the Calvin cycle, a positive cooperative interaction between the active centers of the enzyme subunits is characteristic. The physiological importance of positive kinetic cooperativity with respect to the substrate lies in the fact that, due to it, the range of substrate concentrations necessary for a significant change in the activity of the enzyme decreases [8].
References
- Babadzhanova MA, Nasyrov Yu S (992) Physiology of plants 39(4): 753-759.
- Gontero B, Guidici-Orticoni M, Ricard JA (1994) The Modulation of Enzyme Reaction Rates within Multi-Enzyme Complexes. Eur J Biochem 226(3): 993-998.
- Babadjanova MA, Mirzorahimov AK, Babadjanova MP, Esanalieva Sh A (2010) Plant physiology 57(2): 186-191.
- Karimova MA, Romanova AK (1967) Doman N.G. All-Union Conf. Photosynthesis and the use of solar energy. abstracts. Dushanbe pp. 56-57.
- Babadzhanova MA, Saifudinov AK (2019) Report 62(7): 464-468.
- Kochetov GA (1980) A practical guide to enzymology. Moscow: High school pp. 272.
- Romanova AK (1980) Biochemical methods for studying autotrophy in microorganisms. M: Nauka pp. 160.
- Kurganov BI (1992) Physico-chemical mechanisms of regulation of enzyme activity. M: Nauka p. 62.






























