Fabrication and Photocatalysis Application of Nanoporous Anodic Alumina Formed at Relatively High Temperature
Chen-Kuei Chung* and Chin-An Ku
Department of Mechanical Engineering, National Cheng Kung University, Taiwan
Submission: January 31, 2022; Published: February 10, 2022
*Corresponding author: Chen-Kuei Chung, Department of Mechanical Engineering, National Cheng Kung University, Taiwan
How to cite this article: Chung CK, Ku CA. Fabrication and Photocatalysis Application of Nanoporous Anodic Alumina Formed at Relatively High Temperature. Ann Rev Resear. 2022; 7(1): 555705. DOI: 10.19080/ARR.2022.07.555705
Abstract
The nanoporous anodic aluminum oxide (AAO) is a well-known nanomaterial template that can be used for the synthesis of low-dimensional nanomaterials, as a free-standing membrane or as an etching mask for pattern transfer for various applications. Two-step potentiostatic direct-current anodization (DCA) from high-purity aluminum (Al, ≥99.99%) at low temperature (0-10°C) to avoid Joule-heat enhanced dissolution effect is commonly used for fabricating the AAO template. In contrast, an hybrid pulse anodization (HPA) consisting of the normal-positive and small negative voltages is an effective method to diminish the Joule heating for synthesizing AAO even from the cheap low-purity (≤99.5%) Al foil at a relatively high temperature (HT) of 20-25°C. The pore structure quality of AAO using HPA is better than that using DCA at relatively high temperature due to its effective cooling. The HPA-HT AAO may also be synthesized from the Al coating on high-roughness substrate for more effective surface area to enhance TiO2 photocatalysis performance. The HPA has advantages not only in manufacturing convenience but also for enhancing pore characteristics of AAO at harsh conditions of cheap low-purity Al foils and relatively high temperature for more possibility of application.
Keywords: Nanoporous alumina; AAO; Anodization; Purity; Temperature; Photocatalysi
Abbreviations: AAO: Anodic aluminum oxide; DCA: Direct-current anodization; HPA: Hybrid pulse anodization; HT: High temperature; Dp: Pore diameter; Dint: Interpore distance; w: Wall thickness; tb: Barrier thickness; t: AAO thickness; E: Potential difference; I: Corresponding current; PA: Pulse anodization
Introduction
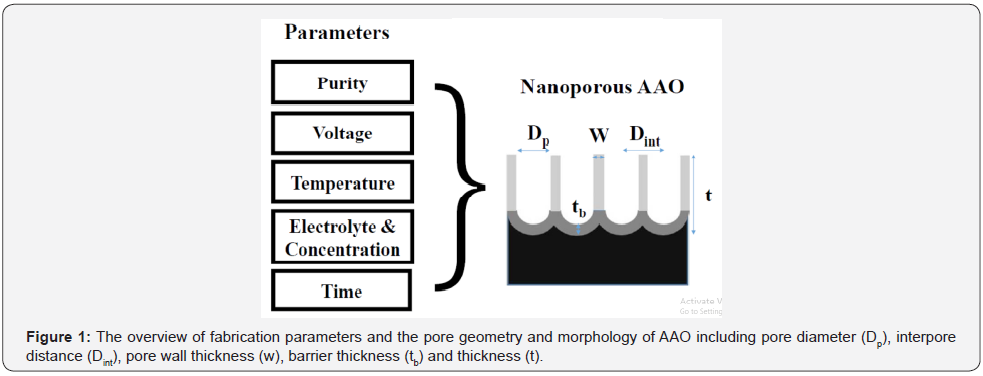
The nanostructured materials with much enhanced physical and chemical properties compared to the bulk ones have attracted much research over these decades. A popular example is the lotus effect that has self-cleaning properties due to the super-hydrophobic property of the leaf with nanostructured surface. The nanoporous anodic aluminum oxide (AAO) material is a significant template that is used for the synthesis of 1D-2D nanomaterials, as a free-standing membrane for filters or as an etching mask for pattern transfer on a desired material in different categories of application. Compared with the porous polymer template i.e., polycarbonate or polystyrene [1,2], AAO has advantages of nanoscale pores, self-organization, controllable pore size and thermal stability [3,4]. Conventional AAO templates are synthesized using two-step direct current anodization (DCA) at low temperature (0-10°C) to avoid dissolution effect. On the contrary, an effective method of hybrid pulse anodization (HPA) with normal-positive and small negative voltages is practical for AAO synthesis at a relatively high temperature of 20-25°C for enhancing performance of AAO structure from the cheap low-purity (≤99.5%), costly high-purity (≥99.99%) aluminum foils and the Al coating on the desired substrates [5-7]. The schematic overview of the process parameter dependent pore geometry and morphology of AAO is shown in Figure 1. The imporatnt factors of pore geometry includes the pore diameter (Dp), interpore distance (Dint), pore wall thickness (w), barrier thickness (tb) and AAO thickness (t) those are linked to diffeernt application. The fabrication parameters like the Al purity, applied voltage, operation temperature, electrolyte content & concentration and time are related to the pore geometry and profile of AAO.
The defects in low-purity Al may increase the dissolution for irregular pore structure. The anodization voltage is proportional to the Dint and generally has a positive relation to Dp. Also, the electrolyte type has its proper anodization voltage to AAO formation. The Titanium dioxide (TiO2) is widely used for photocatalyst after the discovered Honda-Fujishima effect in 1972 [8]. A great number of publications show the research on the microstructure of TiO2 for enhancing performance of photocatalyst. The AAO was studied to assist synthesis of high-order TiO2 nanowire arrays via sputtering and atomic layer deposition for comparison to explore the potential catalysis, and energy applications in the future [9]. Using optoelectronically engineered nanoporous photonic crystals for visible light-enhanced photocatalysis is proposed recently [10]. In addition, the HPA at a relatively high temperature is useful for anodizing the high-roughness Al film with hillocks or spheres morphology to form the modified 3D AAO that is advantageous for the following TiO2 film or nanoparticles coating for enhancing the the photocatalysis enhancement owing to the increased contact surface area [7,11].
Discussion
Fabrication of AAO at Relatively High Temperature
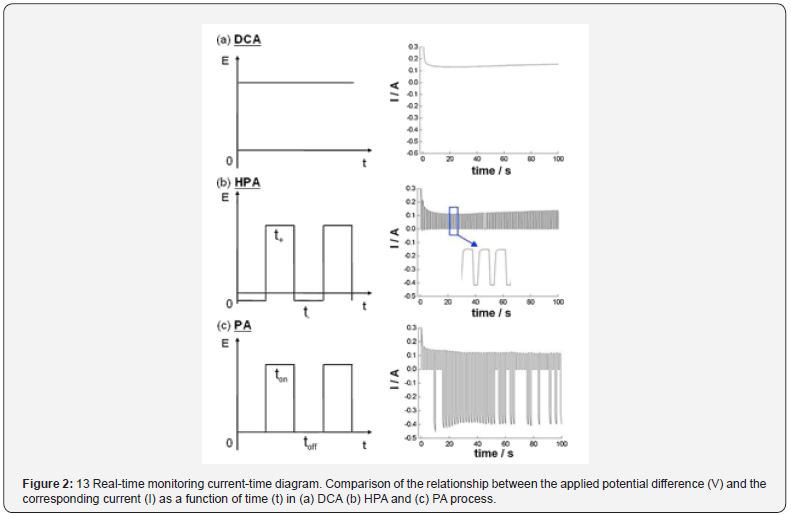
The traditional two-step direct-current anodization (DCA) on Al foils at a constant voltage may lead to the well-ordered AAO configuration compared to the one-step anodization. However, the stern process conditions are usually required at low temperatures of 0-10°C from high-purity (>99.99%) Al foils. The low electrolyte temperature is to reduce the Joule-heat induced dissolution rate of alumina during anodization but corresponds to the decreased growth rate. On the other hand, Chung et al. [5,6] demonstrated the feasibility of AAO fabrication from low-purity Al foil (≤99.5%) at relatively high temperature (HT) of 20-25°C using the hybrid pulse anodization (HPA) method consisting of the normal-positive and small negative voltages that could overcome the limitation of conventional low-temperature AAO process to form nanopores by diminishing Joule heat during anodization, even from the cheap low-purity Al material. Figure 2 shows the real-time monitoring current-time diagram of the relationship between the applied potential difference (E) and the corresponding current (I) as a function of time (t) in (a) DCA (b) HPA and (c) PA (pulse anodization) process at 25°C, repectively. The continuous I-t diagram in DCA (Figure 2a) leads to heat accumulation for the thermally enhanced Joule heat dissolution to break the pore structure formed at 25°C. In contrast, the pore structure formed by HPA at 25°C can be kept due to the effective cooling of tiny current during t- duration to diminish the Joule-heat dissolution (Figure 2b). Regarding the I-t relation by conventional pulse anodization (PA) with alternate positive and zero potential differences (Figure 2c), we can see the clear negative current during PA compared to the nearly zero current during HPA. In the toff duration of PA with 0 V, it is like a discharging capacitor to randomly release the charges accumulated in ton duration for the cathodic current that is attributed to the impurities of Al and the quick change of polarization between two electrodes in a very short time. For the t- duration of HPA with a small value, it can reduce the discharging effect and have insufficient potential to overcome the energy barrier height of cathodic reduction reaction. Therefore, nearly zero cathodic current occur accordingly.
Application of AAO for enhancement of TiO2 photocatalysis
The case study of nanoporus AAO template applied for enhanceing TiO2 photocatalysis is that using the commercial P25-TiO2 nanoparticles (21±5 nm) coating by sol-gel method on the various HPA AAO films on the Si substrates. The formation of HPA AAO pores on the smooth (2D) and extremely rough (3D) Al films can produce the 2D planar and 3D AAO nanostructures, respectively. The 3D AAO is also over-etched in the 5 wt% phosphoric acid at 25°C during pore widening and called as the over-etched 3D nanoporous AAO film. Figure 3a-c shows the SEM micrographs of the commercial P25-TiO2 nanoparticles coating on the 2D AAO, 3D AAO and over-etched 3D AAO films, respectively. It is seen that the TiO2 nanoparticles are partly adsorbed on the cell walls of each pore in the planar AAO due to the van der Waals force. Also, less P25-TiO2 nanoparticles diffuse into the pore channels (Figure 3a). The nanoparticles in the solution are considered as Brownian motion for coating on the AAO film. The random motion results in difficulty of P25-TiO2 nanoparticles to move into the single-aspect pore channel of planar AAO. On the other hand, the 3D porous AAO exists more TiO2 nanoparticles adsorption not only on the surface but also in pore channels (Figure 3b). The hillocks in rough surface containing a great number of non-perpendicular pores for more probability of introducing P25-TiO2 nanoparticles in Brownian motion.

Moreover, the valleys in the 3D AAO structure are helpful to aggregate more TiO2 nanoparticles comparing to the planar structure. Figure 3c shows the P25-TiO2 nanoparticles deposited on over-etched porous AAO by longer pore widening. It reveals the most TiO2 nanoparticles appear in the very rough substrate. Comparing with the 3D AAO structure, the over-etched porous AAO has much larger pore size and surface area to accommodate and adsorbed more P25-TiO2 nanoparticles. In addition, the P25-TiO2 nanoparticles adhesion in the pore channels are much stable than on the surface that benefits for photocatlysis. The photocatalytic performance of P25-TiO2 on various supporting materials including Si wafer, 2D AAO, 3D AAO, and over-etched 3D AAO after water flushing is further studied. For the as-deposition samples, the phtocatalysis data show similar decomposition trend either in the pure Si wafer or AAO (over-etched 3D). Each sample exhibits good phtocatalytic performance by completely decomposing MB after 12 h UV irradiation. However, the P25- TiO2 on the pure Si behaves greatly decrease in phtocatalysis after water flushing. After 20 h UV irradiation, the concentration of MB maintains in 82.3%. It reveals that desorption of P25-TiO2 from the supporting Si wafer happens due to the poor adhesion. In contrast, the MB concentration decreases to 22.6% for the overetched 3D AAO sample after 20 h UV irradiation. It evidences the good adhesion of P25-TiO2 on the AAO. The over-etched 3D AAO has the best photocatalytic performance towards degradation of MB due to the largest surface area for photocatalysis reaction and adsorption of TiO2. It is noted that the 2D AAO and 3D AAO have inbetween photocatalysis performance with 55.7% and 48.8% MB concentration after 20 h photo-degradation. This implies that the P25-TiO2 which adsorbed on 2D AAO and 3D AAO surface slightly desorbed after to water flushing. However, the porous structure can keep most of P25-TiO2 in pores comparing to the flat surface of Si wafer.
Conclusion
The HPA with normal-positive and small negative voltages is an effective method for AAO fabrication from low-purity Al at relatively high temperatures of 20-25°C for promoting AAO structure in comparison with conventional AAO synthesized from high-purity Al using two-step DCA at low temperature (0-10 °C) to avoid dissolution effect. Comparing the I-t curves of DCA and HPA from bulk Al, the HPA at the negative period with nearly zero cathodic current can exhibit effective cooling to diminish Joule heating for promoting AAO quality. In addition, the HPA AAO can be synthesized at 25°C from the Al coating on high-roughness substrate for more effective surface area and good adhesion to enhance TiO2 photocatalysis performance after water flushing. The best photocatalysis performance can be obtained by depositing commercial P25-TiO2 nanoparticles on the over-etched 3D AAO. After water flushing, the over-etched 3D AAO maintains the ~77% phtocatalysis performance of as-deposited sample. Moreover, the over-etched 3D AAO exhibits the largest surface area for catalytic reaction and adsorption of p25-TiO2 compared with the intact 2D and 3D AAO.
Acknowledgement
This work is partially sponsored by the Ministry of Science and Technology (MOST), Taiwan, under contract No MOST 106- 2221-E-006 -101 -MY3 and 110-2221-E-006-177.
References
- Kim BS, Kim HJ, An S, Chi S, Kim J, et al. (2017) Micro- and nano-porous surface pattern prepared by surface-confined directional melt crystallization of solvent. Journal of Crystal Growth 469: 184-190.
- Long M, Peng S, Chen J, Yang X, Deng W (2016) A new replication method for fabricating hierarchical polymer surfaces with robust super hydrophobicity and highly improved oleophobicity. Colloids and Surfaces A: Physicochem Eng Aspects 507: 7-17.
- Liu CL, Chen HL (2018) Crystal orientation of PEO confined within the nanorod templated by AAO nanochannels. Soft Matter 14: 5461-5468.
- Wei QL, Fu YQ, Zhang GX, Yang DC, Meng GW, at al. (2019) Rational design of novel nanostructured arrays based on porous AAO templates for electrochemical energy storage and conversion. Nano Energy 55: 234-259.
- Chung CK, Zhou RX, Liu TY, Chang WT (2009) Hybrid pulse anodization for the fabrication of porous anodic alumina films from commercial purity (99%) aluminum at room temperature. Nanotechnology 20(5): 055301.
- Chung CK, Chang WT, Liao MW, Chang HC, Lee CT (2011) Fabrication of enhanced anodic aluminum oxide performance at room temperatures using hybrid pulse anodization with effective cooling. Electrochimica Acta 56: 6489-6497.
- Chung CK, Tu KT, Chang CY, Peng YC (2019) Fabrication of thin-film spherical anodic alumina oxide templates using a superimposed nano-microstructure. Surface & Coatings Technology 361: 170-175.
- Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238: 37-38.
- Yao Z, Wang C, Li Y, Kim NY (2015) AAO-assisted synthesis of highly ordered, large-scale TiO2 nanowire arrays via sputtering and atomic layer deposition. Nanoscale research letters 10: 1-7.
- Lim SY, Hedrich C, Jiang L, Law CS, Chirumamilla M, et al. (2021) Harnessing Slow Light in Optoelectronically Engineered Nanoporous Photonic Crystals for Visible Light-Enhanced Photocatalysis. ACS Catalysis 11: 12947-12962.
- Chung CK, Liao MW, Kuo EH, Wang ZW (2018) Enhancement of photocatalytic performance of P25‐TiO2 nanoparticles by 3D nanoporous anodic alumina at room temperature. International Journal of Applied Ceramic Technology 15: 438-447.






























