ActiGraft Treatment in Complex Wounds with Exposed Structure - A Case Series
Maxim Gurevich1, Naz Wahab2, Chinenye Wachuku3, Karim Ead J4 and Robert J Snyder5*
1Diabetic Foot Clinic, Hillel Yaffe Hospital, Hadera, Israel
2Wound Care Experts, Las Vegas, NV, USA
3Hoboken University Medical Center, Hoboken, New Jersey, USA
4Westside Reginal Medical Center, Florida, USA
5Barry University, 7301 N University Drive, 33321 after Florida, USA USA
Submission: October 20, 2021; Published: November 08, 2021
*Corresponding author: Robert J Snyder, Barry University, 7301 N University Drive, 33321 after Florida, USA
How to cite this article: Gurevich M, Wahab N, Wachuku C, Ead KJ, Snyder RJ. ActiGraft Treatment in Complex Wounds with Exposed Structure - A Case Series. Ann Rev Resear. 2021; 7(1): 555701. DOI: 10.19080/ARR.2021.07.555701
Abstract
Introduction: Limb amputation as a result of non-healing complex wounds continues to be with high prevalence. A non-healing wound can deteriorate and have an extensive breakdown of soft tissue that may cause exposure of vital structures. ActiGraft, an autologous whole blood clot, acts as a protective scaffold and mimics the extracellular matrix properties, promotes cells granulation, and creates a balance of moisture in the wound area, supporting the healing process.
Patients and Methods: All patients signed informed consent prior to their participation in the study. Complex wounds with exposed bone or tendon, free of infection were treated with ActiGraft application as part of a Registry study (NCT04699305).
Results: ActiGraft treatment in post Transmetatarsal amputation (TMA) DFU wound, venous ulcer and pressure ulcer increased wound granulation resulted in coverage of vital structures and reduction in wound area. All patients suffered from multiple comorbidities and were treated previously with multiple treatments that failed to progress the wound. The number of applications varies from 1 application and 11 applications and an overall treatment duration of 8 weeks and 23 weeks respectively.
Discussion: ActiGraft is a point of care treatment for safe and rapid preparation, using the patient’s peripheral blood to create a whole blood matrix. ActiGraft treatment in complex wounds with exposed bone and tendon showed to promote cell granulation and progress the wound toward healing.
Keywords: ActiGraft; Autologous Whole Blood Clot; Extracellular Matrix; Complex Wounds; Exposed structures
Abbreviations: TMA: Transmetatarsal amputation; DFU: Diabetic foot ulcers; ECM: Extracellular matrix; ACDA: Acid Citrate Dextrose Adenine; RA: Rheumatoid arthritis; DR: Dynamic reciprocity; ABCT: Autologous whole blood clot-based therapies
Introduction
Diabetes mellitus continues to present as a huge burden on the healthcare system and is a leading cause of diabetic foot ulcers (DFUs). 85% of all limb amputations are secondary to a recent DFU [1]. Due to the increasing rate of DFUs, there is a discouraging reality of a heightened amount of total, major, and minor amputations as a means of treating severe and nonhealing ulcerations. Interestingly, the most affected population tends to be young (18–44 years old) and middle-aged (45–64 years old) adults [2]. DFUs in many cases are hard to manage as a result of a compilation of risk factors, including the presence of infection, external trauma, neuropathy, peripheral arterial disease, poor hygiene, and malnutrition, among others.
Complex wounds, secondary to poorly managed ulcerations, infection, or surgical intervention, often make these conditions difficult to manage. Wounds associated with patients who are diagnosed with medical conditions and have comorbidities tend to take on worsening characteristics over time. Many times, the wound may appear fibrotic, have a slough, or may even become necrotic. With a lack of healing, an extensive breakdown of soft tissue may cause exposure of bones, tendons, and other vital structures, resulting in the need for a more aggressive treatment approach and often will necessitate inpatient admission to the hospital. The loss of critical components within the wound bed such as the extracellular matrix (ECM) in combination with exposed structures can significantly impact treatment pathways and prolong healing time. With the deterioration of the ECM, a natural development of granulation tissue and subsequent epithelization over vital structures becomes extremely slow and may not occur at all [3].
Due to the lack of structure within the ECM, organized healing cannot take place because of the lack of chemoattractant signals from the ECM to recruit growth factors, chemokines, and mediators to the injured area. The healing process in those wounds occurs in layers as a result of the extensive deep nature of the deficit. Deep tissue reconstruction must mimic the normal morphology found beneath the dermis, replacing and revascularizing the matrix to ensure a wound environment in which healing may occur [3]. One of the greatest challenges in treating complex wounds, is that many times, the current treatment options cannot be utilized as monotherapy for granulation of the wound bed and usually require some assistance in the form of a tissue scaffold to support the migration of tissues over the deep soft tissue deficit and poorly vascularized wound bed [4]. The majority of options that can be utilized may not always be beneficial to the wound area for many reasons such as immunologic rejection of the treatment. Time is also an important factor that may deem the treatment modality unsuccessful due to the decreased rate at which healing occurs. Recent studies have demonstrated that a wound is less likely to heal by 12 weeks if there is little reduction in wound area (percent area reduction) during the first 4 weeks of care [5].
Many times, providing treatment for a long period of time may not be a sufficient option because the wound will continue to worsen after 4 weeks if there has been no improvement due to the lack of a proper environment sufficient for healing. The treatment option utilized may only stall further damage from occurring to the wound bed, but it will still necessitate another form of treatment that may work effectively and in a timely manner. Another factor that may limit wound healing is the availability and maintenance requirements for the treatment option that is not only efficacious but also cost-effective. Clinicians must take into consideration a treatment that can be utilized in all forms of chronic and complex wounds to promote wound healing, provide coverage of exposed vital structures, whilst having the ability to deter immunological rejection from the body.
ActiGraft is an autologous whole blood clot, utilized in an outpatient setting for safe and rapid preparation of a whole blood clot matrix. ActiGraft acts as a protective scaffold and mimics the ECM properties, supporting the wound healing process and the progression from an acute phase to the proliferative phase.
ActiGraft was found to promote cell granulation and to provide a supportive and optimal environment for wound healing. The autologous whole blood clot creates a balance of moisture to the wound area, along with lowering the pH levels in the wound area, achieving a more optimal pH of between 7.2 and 7.6, which was shown to promote epithelialization whilst mediating cellular migration and proliferation of keratinocytes and fibroblasts, stimulation of growth factors, angiogenesis, collagen synthesis, along with and coverage of the wound bed [6,7] which facilitates the migration of fibroblasts and keratinocytes, thus producing a faster closure.
Materials and Methods
Patients
Patients, 18 years or older, exhibit complex wounds with exposed bone or tendon, signed the informed consent agreeing to use their medical history data, demographics, and wound characteristics including wound images. The patients are part of a registry study (NCT04699305).
Wound Bed Preparation
Prior to ActiGraft application, all wounds underwent wound bed preparation of complete debridement of the wound tissue, bacterial balance, and moisture balance. Debridement was performed using surgical enzymatic, autolytic, or mechanical methods and was widely used to clear necrotic tissue. Any signs of infection, characterized clinically by signs of color changes in the wound bed, friable and unhealthy granulation tissue, abnormal odor, increased serous exudate, and pain at the wound site were assessed in each visit along with moist balance, avoiding and excessive periwound moisture that might cause skin maceration. A secondary dressing change was performed to keep the wound dry and to prevent maceration [8,9].
ActiGraft Application and Procedure
Blood was withdrawn from the patient into Acid Citrate Dextrose Adenine (ACDA) vacuum tubes at a point of care. The blood was then injected into a coagulation mold. Calcium coagulant and kaolin were added in order to start the coagulation process. Following the procedure, the blood was allowed to clot for 8-12 min. The clot was gently removed from the coagulation mold and was attached to the wound bed, using steri strips. A non-adherent pad was placed over the clot covered with a secondary dressing.
Results
Case 1
A 68-year-old female with type-2 diabetes mellitus (T2DM) and a medical history that includes peripheral neuropathy, End- Stage Renal Disease, and peripheral vascular disease, exhibited with gangrene of the great toe with extensive infection noted to the left foot. An attempt for a first ray amputation took place, however, due to the extensive infection it failed. A transmetatarsal amputation was performed and left open to heal via secondary intention. Past treatments included negative pressure wound therapy and hyperbaric medicine. However, due to the complex nature of this wound, the patient required an advanced dressing that incorporates robust ECM technology to help manage each phase of the wound healing process. Prior to the initial application of ActiGraft, the wound consisted of a fibrogranular wound base with minimal necrotic tissue noted to the proximal aspect of the wound bed. Exposure of bone and edema to the plantar surgical flap was noted. ActiGraft was introduced to the wound on day 1. Due to the patient’s comorbidities, the patient was admitted to the hospital for 3 weeks before returning to the clinic for follow-up. During the patient’s hospitalization, the wound was treated with Xerofom. The patient returned for a follow-up visit on week 4, exhibiting massive granulation over the bone. A standard of care treatment was applied thereafter. On week 8, the wound showed an area reduction of 80%. The minimally exposed wound bed was consistent with healthy granulation tissue with ingrowth of soft tissue layers leading to coverage of vital structures such as the previously exposed bone. Wound healing had occurred, allowing for the growth of healthy skin to take place (Figure 1).
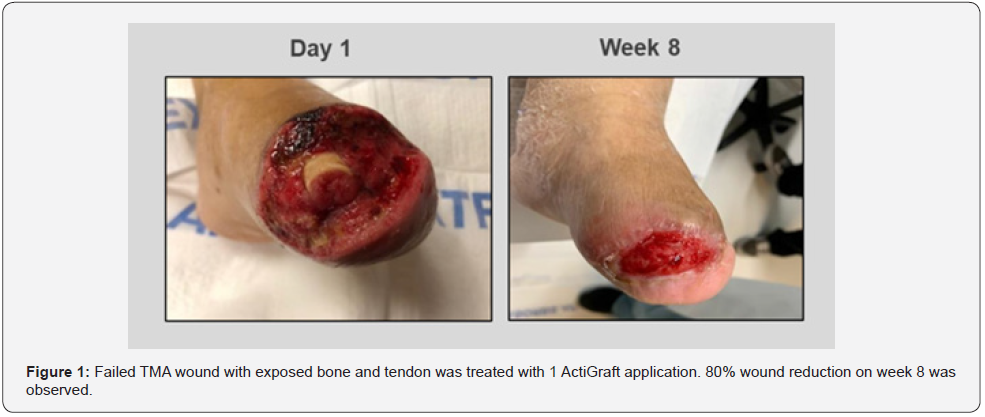
Case 2
A 79-year-old female presented with a chronic, 1-month-old, non-healing venous leg ulceration with exposed bone, positioned on the anterior aspect of the patient’s left leg. The patient had a past medical history of chronic venous insufficiency, hypertension, anemia, osteoporosis, hyponatremia, polymyalgia rheumatic, colostomy, diverticulosis colon, and rheumatoid arthritis (RA). The patient was treated with steroids and Methotrexate for her RA. Initial wound examination revealed full-thickness ulceration that spanned the anterior aspect of the left leg. Wound borders were irregular with a violaceous periwound environment. The wound bed consisted of a 90/10 mix of fibrotic nonviable tissue with minimal granulation. Although the patient exhibited symptoms of venous insufficiency, treatments failed to improve the lesions and her wounds continued to worsen. Local wound management with a silver-impregnated foam dressing and selective debridement failed to garner improvement. However, once the wound failed to demonstrate signs of progress, ActiGraft was introduced to the wound resulting in complete healing after 14 weeks with only 4 applications (Figure 2).
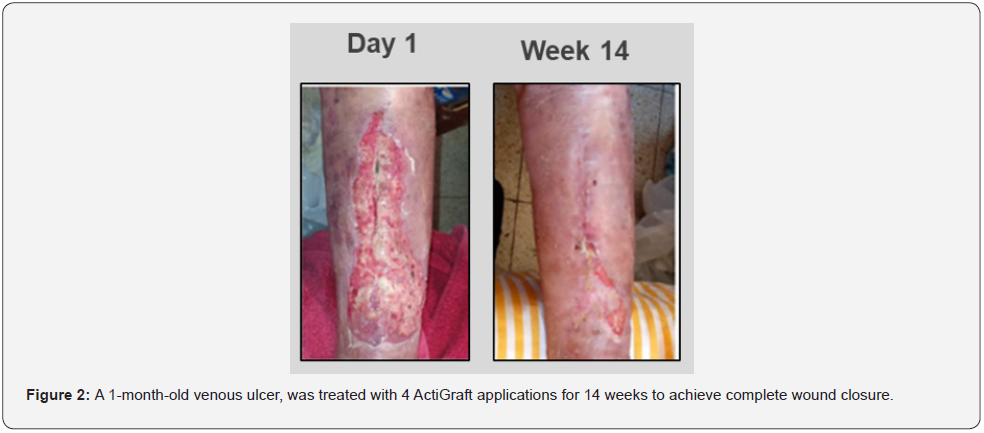
Case 3
A 57-year-old male exhibited a pressure ulcer on the left heel with exposed bone. The patient suffers from severe peripheral vascular disease, T2DM, arterial insufficiency, severe COPD, renal insufficiency, and upper gastrointestinal bleeding. The patient underwent an above-knee amputation on the right lower extremity, after a cerebrovascular accident. The wound was treated for osteomyelitis and a partial calcanectomy was performed to remove the infected bone tissue. Negative pressure therapy was utilized to treat the wound but resulted in no signs of improvement. Advanced dressings were utilized in attempts to control wound deterioration along with repeated ultrasonic debridement and the sharp debridement of the wound. A belowknee amputation was suggested at this point, however, the patient refused to undergo another limb amputation. Prior to the first application of Actigraft, the wound consisted of a fibrogranular base with necrosis and exposed tendon and bone. There was hyperkeratotic tissue surrounding the wound bed noted. After week 1 of ActiGraft application, the wound was reduced by 34.6% showing major improvement in all the wound characteristics. ActiGraft treatment reduced the wound area by 88.77% by week 12. Use of the autologous whole blood clot was continued afterward with a total of 11 applications over the period of 23 weeks (at the middle the treatment was interrupted due to acute COVID-19 infection of the patient ) resulting in a complete wound healing and coverage of exposed vital structures (Figure 3).
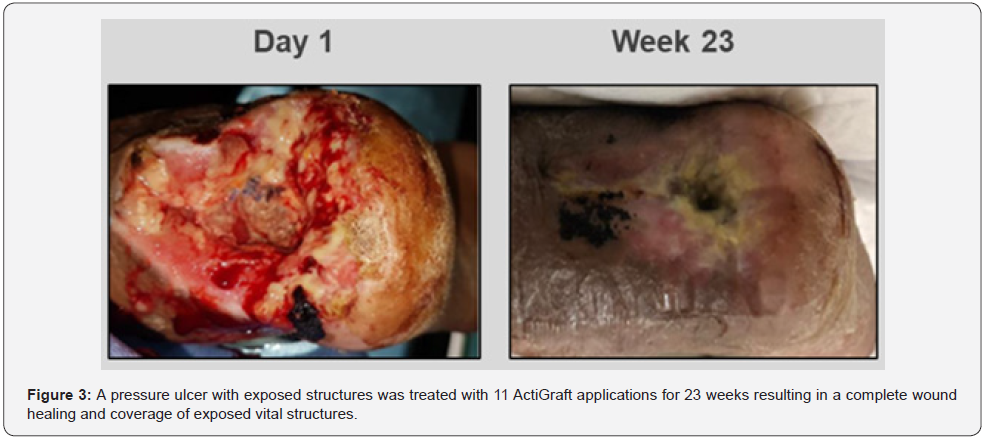
Discussion
Wound healing represents a comprehensive series of wellorchestrated physiological reactions that create an orderly healing cascade. This process includes four key phases: hemostasis, inflammation, proliferation, and remodeling [10]. It progresses along a continuum that should result in the restoration of anatomical and functional integrity. Despite the etiological differences in chronic wounds, they often share common pathophysiologic features. It is vital for clinicians to utilize advanced wound dressings that assist in activating the body’s intrinsic healing potential. The evolution of wound care therapies has targeted regenerative medicine and the use of biologic scaffolds for their potential benefit on recalcitrant and difficultto- heal complex wounds. Schultz et al defined dynamic reciprocity (DR) as an ongoing, bidirectional interaction amongst cells and their surrounding microenvironment [11]. This phenomenon may help elucidate how various biochemical abnormalities fit together and their disruptions in one part of the wound healing cascade may lead to disruptions in subsequent interactions that ultimately prevent chronic wounds from healing.
Hence, defects in the early stages of wound healing, may have downstream effects that eventually preclude wound closure. To mitigate and harmonize DR within the chronic wound ecosystem, autologous whole blood clot-based therapies (ABCT) can modulate each stage of the wound healing cascade [12]. The scaffold created by the autologous whole blood clot provides an environment that is favorable for dynamic macrophage plasticity to take place. Through proper cytokines and mediators, M1 macrophages can transform from their pro-inflammatory phenotype into M2 the anti-inflammatory phenotype [12]. This scaffold provides a medium in which the body can transform the wound from a non-healing chronic condition into a healing “acute” condition. This mesh promotes cellular adherence, modulates cell function, and serves as a reservoir for growth factors, proteases, and protease inhibitors. Additionally, these advanced dressings can help potentially lower bacterial bioburden while stimulating angiogenesis and fostering the movement of keratinocytes and fibroblasts to ultimately aid in wound contracture.
ActiGraft was found to provide an effective treatment measure that negates rejection from the body when the application of the graft takes place. The autologous nature of this treatment deters any negative implications that may arise due to immunologic rejection. This modality is tailored specifically to the patient and enhances their natural healing mechanisms by utilizing elements derived from the body’s own habitat. ActiGraft is transformed into a scaffold for the reconstruction of the ECM, providing protection and coverage for wound healing to occur while bringing forth an organized manner for adhesion of growth factors, cytokines, and mediators to work in tandem to progress through the wound healing phases, promoting the secretion of growth factors and assisting with revascularization and epithelization of the wound bed [12].
Conclusions
ActiGraft can be applied in an outpatient setting, preventing the need for hospital admission with no limitation to wound location or neurovascular structures in the surrounding area. ActiGraft provides simplicity and preparation in a short period of time at the patient’s bedside, increasing the potential for patient compliance. The ActiGraft treatment presents a high safety pattern. Incorporating treatment with the use of the ActiGraft in complex wounds with exposed bone and tendon showed promotion of cell granulation and progression of the wound towards healing. Depending on the depth of the skin deficit, this granulation may completely close the wound bed or can be utilized as preparation for future skin grafts to be applied. Closure of the wounded area is achieved efficiently by enhancing the body’s physiological means of advancing through the wound healing process in a natural way.
References
- Mavrogenis AF, Megaloikonomos PD, Antoniadou T, Igoumenou VG, Panagopoulos GN, et al. (2018) Current concepts for the evaluation and management of diabetic foot ulcers. EFORT Open Rev 3(9): 513-525.
- Geiss L, Li Y, Hora I, Albright A, Rolka D, et al. (2018) Resurgence of Diabetes-Related Nontraumatic Lower-Extremity Amputation in the Young and Middle-Aged Adult U.S. Population. Diabetes Care 42(1): 50-54.
- Simman R, Hermans M (2017) Managing Wounds with Exposed Bone and Tendon with an Esterified Hyaluronic Acid Matrix (eHAM): A Literature Review and Personal Experience. J Am Coll Clin Wound Spec 9(1-3): 1-9.
- Negut I, Dorcioman G, Grumezescu V (2020) Scaffolds for Wound Healing Applications. Polymers (Basel) 12(9): 2010.
- Snyder RJ, Cardinal M, Dauphinée DM, Stavosky J (2010) A post-hoc analysis of reduction in diabetic foot ulcer size at 4 weeks as a predictor of healing by 12 weeks. Ostomy Wound Manage 56(3): 44-50.
- Emre O, Chinenye WM (2021) The Use of ActiGraft, an Autologous Skin Graft, in the Treatment of Complex Diabetes Foot Ulcer - A Case Study. Ann Rev Resear 6(2).
- Vallejo L, Achterberg J (2020) Uso de una matriz autóloga en el tratamiento de úlceras de pie diabético, con espectroscopia de infrarrojo cercano y medidor de pH dé J Wound Care 29(LatAm sup 3): 24-31.
- Mat Saad AZ, Khoo TL, Halim AS (2013) Wound bed preparation for chronic diabetic foot ulcers. ISRN Endocrinol 2013: 608313.
- Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, et al. (2003) Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 11(Suppl 1): S1-28.
- Fitridge R, Thompson M (2011) editors. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]. Adelaide (AU): University of Adelaide Press.
- Schultz G, Davidson J, Kirsner R, Bornstein P, Herman I (2011) Dynamic reciprocity in the wound microenvironment. Wound Repair and Regeneration 19(2): 134-148.
- Snyder RJ, Schultz G, Wachuku C, Rashid AM, Ead JKK (2020) Proposed Mechanism of Action of Topically Applied Autologous Blood Clot Tissue: A Quintessential Cellular and Tissue Based Therapy. J Am Podiatr Med Assoc 13: 20-140.






























