Puerto Ricans Show Higher Prevalence of MTHFR C677T and A1298C Mutations and Higher Risk of Thrombophilia Even Without Hyperhomocysteinemia: A Preliminary Survey
Morales Borges RH1*, Pagan Lugo MA2, Martínez Osorio GS3, Ramírez BN2, Carlo Torres SE4, González MJ5, Duconge J6, Disdier OM7and Marlon CE8
1Integrative Optimal Health of Puerto Rico, USA
2University of Science, Arts and Technology, USA
3Universidad Central del Este, Dominican Republic
4Ponce School of Medicine, USA
5Department of Public Health, University of Puerto Rico, USA
6Department of Pharmaceutical Sciences, University of Puerto Rico, USA
7Puerto Rico Institute of Statistics, USA
8The Funding Network Puerto Rico, USA
Submission: June 03, 2018;Published:June 27, 2019
*Corresponding author: Raul H Morales Borges, Integrative Optimal Health of Puerto Rico and New Alliance Integrative Research, San Juan, PR Address: 29 Washington St. Suite # 107, San Juan, Puerto Rico, 0907-1509, USA
Abstract
The methylenetetrahydrofolate reductase (MTHFR) mutation is associated with increased risk of myocardial infarctions, stroke, venous thrombosis, recurrent pregnancy losses between other thrombophilia’s. In our practice we see arterial and venous thromboembolism with no hyperhomocysteinemia, and we see more cases of recurrent pregnancy loses. That is one reason we are proposing this study.
Very high homocysteine levels rarely result from having two common variants alone. People with very high homocysteine levels should be carefully evaluated for other factors known to affect homocysteine. It has been studied high frequencies and prevalence’s in Europeans, Mediterranean’s, and Hispanics, but in general high homocysteine levels associated with these mutations have been related to be thrombogenic, although in our practices we don’t see that.
A cross-sectional assessment of medical records of patients with MTHFR polymorphisms (carriers) within Puerto Rico from multiple physicians’ referrals was made. We reviewed 2752 active records of which 101 cases had the genetic mutation test positive for MTHFR for a prevalence of 3.67% of which only 13.9% had hyperhomocysteinemia although 28.7% had deep venous thrombosis and 12.9% had stroke. We recognized it was a small sample and this is a reason that a larger prospective study is needed.
Introduction
The methylenetetrahydrofolate reductase (MTHFR) is coded by the gene with the symbol chromosome 1 location p36.3 in humans and there are DNA sequence variants (genetic polymorphisms) associated with this gene, although the two most common ones are C677T and A1298C [1]. MTHFR tells our body how to create an enzyme involved in breaking down the amino acid homocysteine [2-4].
Deficiencies in production or function of this enzyme have been associated with increased risk of myocardial infarctions, stroke, venous thrombosis, several types of cancer congenital defects, inflammatory bowel disease, and several neuropsychiatric conditions.
Studies have found that women with two C677T gene variants have an increased risk for having a child with a neural tube defect [5]. Studies have also found that men and women with two C677T gene variants and elevated homocysteine levels may be at a mild increased risk for blood clots (venous thromboembolism) [5]. In our practice we see arterial and venous thromboembolism with no hyperhomocysteinemia, and we see more cases of recurrent pregnancy loses. That is one reason we are proposing this study.
Very high homocysteine levels rarely result from having two common variants alone. People with very high homocysteine levels should be carefully evaluated for other factors known to affect homocysteine. Doing so may bring to light dietary deficiencies, thyroid disease, diabetes, high cholesterol, or lifestyle factors (physical inactivity, smoking and obesity) which can impact, although it was confirmed that C677T is associated with high homocysteine levels. If high levels of homocysteine cannot be explained by these factors, a consultation with a genetics professional may be helpful in identifying rare genetic causes of the high homocysteine [6].
It has been found at high frequencies in Europeans and American Caucasian population [7]. C677T polymorphism shows a wide regional and ethnic variation. Homozygosity (TT) among Whites is 6-14%. In African populations and in Blacks living outside of Africa such as in Brazil and in the United States, the frequency falls to less than 2% for the TT variant [8]. The prevalence rises in Mediterranean and Hispanic population [9]. For example, among Hispanics in prevalence ranges as high as 21% [8]. As per other reports, in America, about 25% of people who are Hispanic, and 10-15% of people who are Caucasian have two copies of C677T [5].
The A1298C mutation, on the other hand, does not show as much population variance; its prevalence is more uniform within the currently studied groups. One study demonstrated that the high frequencies of the C677T/C677T and C677T/ A1298C diplotypes and haplotypes that are found in Hispanics may contribute to a wide spectrum of anomalies, especially in population subject to poor nutrition and low folate intake [10].
A cross-sectional assessment of medical records of patients with MTHFR polymorphisms (carriers) within Puerto Rico from multiple physicians’ referrals was made.
Materials and Methods
The aim of this project was to:
a) Confirm the hypothesis that Puerto Ricans are more prone than general population in the published medical literature regarding venous and arterial thromboembolism with either mutations A1298C or C677T
b) Prove that Puerto Ricans seems that do not need to have a documented hyperhomocysteinemia to have a thrombotic event.
We prepared a formulary to collect the data from the medical records of subjects identified by their physicians collaborating with the study and a questionnaire for the prospective ones identified. Once the data was collected a prevalence estimation (frequency distribution of MTHFR variants and their corresponding diplotypes/haplotypes) and comparison of the observed prevalence versus that reported in reference populations from the 1000 Genome project [11] (e.g. Puerto Ricans, Hispanics, Caucasians, African Americans, etc.) in the study population was performed followed by critical analysis with articles identified after medical literature reviewed. The possible barrier we anticipated is the participation of physicians as volunteers in the study providing their medical records for review. The study involved human ethics and HIPAA Law due to patients participating either their medical records or answering the questionnaire.
We wanted to determine how important and what clinical value is to perform the genetic mutations of MTHFR in our population to identify the ones at risk. To prevent cerebrovascular, cardiovascular, and obstetric thrombotic events. To teach the physicians and health professionals to do this test more regularly. To allow health insurance companies to cover this test. It will be interesting if more Hispanic population can be included in a near future such as subjects from Dominican Republic, Virgin Islands, Central and South America.
There was evidence that we need to do this study in Puerto Ricans and Hispanics [12-16] and to do comparison with prevalence studies in humans with other races [8,9,17,18].
Results
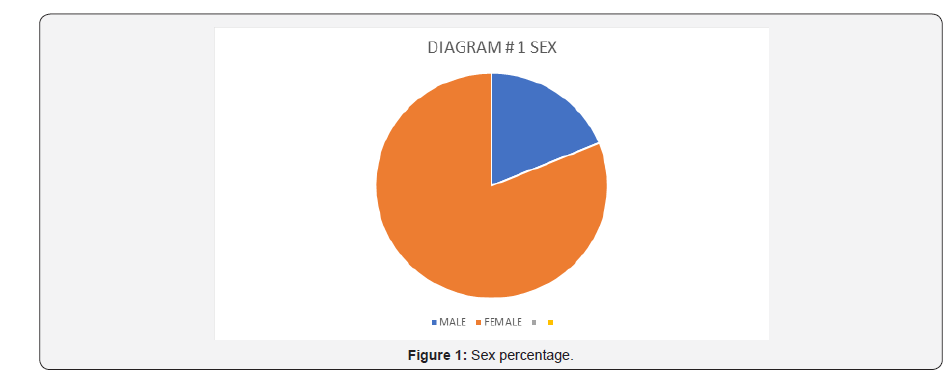
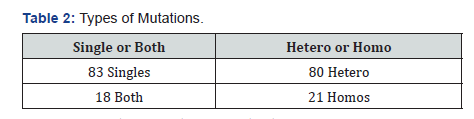
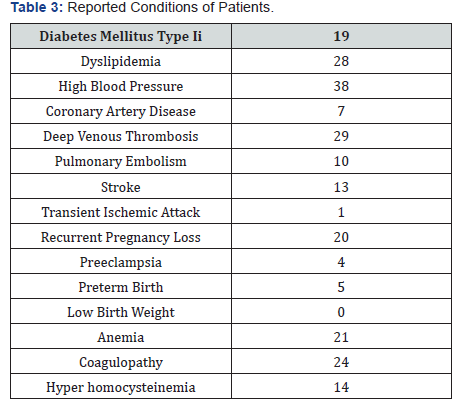
We reviewed 2752 active records of which 101 cases had the genetic mutation test positive for MTHFR for a prevalence of 3.67% (Table 1). Of those cases 81.2% were females and 18.8% males. Twenty-eight percent of the cases had dyslipidemia, 37.6% with hypertension, and 18.8% were diabetics. Around 79.2% were heterozygous and 21% homozygous, 83% were single, and 13.9% had hyperhomocysteinemia. Twenty-one percent of the cases had anemia, 28.7% with deep venous thrombosis, 12.9% with stroke, 0.07% with coronary artery disease, 0.04% with pre-eclampsia, and 0.05% with premature births (Figure 1 & 2) and (Table 2 & 3).


Discussion
One study making a relationship with a risk of colorectal carcinoma (CRC) and MTHFR mutations revealed differences in the rate of MTHFR gene polymorphism mutations and the associated risks with CRC across different global populations. Geographical location played an important role in the rate of MTHFR gene polymorphisms [19]. This is one reason we need to do further studies globally to determine the exact expression of those mutations in different populations.
In our analysis the prevalence was lower than in reported studies from medical literature. This was a small study sample and it could be the reason of low prevalence, although a high-risk population was identified such as patients with Diabetes mellitus type II, High Blood Pressure, and Dyslipidemia. Interestingly we identified venous thrombo-embolisms more frequent than cardiovascular and arterial disease. One fourth of the cases presented with anemia which can be related to MTHFR mutation.
C677T mutation was higher ten A1298C as described in published papers before. Our hypothesis regarding hyperhomocysteinemia was confirmed that Puerto Ricans do not have to express high homocysteine levels to have thrombotic or ischemic events.
I agree with Prospective studies of Hispanic women with these mutations and pregnancy outcomes will establish if there is a causal relationship
Conclusion
We reviewed 2752 cases of which 101 had the MTHFR mutation for a prevalence of 3.67% of which 13.9% had high homocysteine levels. Almost one third of the cases have thrombophilia, so, we demonstrated that Puerto Ricans have a higher prevalence of MTHFR mutations with higher risk of thrombophilia despite having normal homocysteine levels. Larger sample study is recommended.
References
- Björndahl L, Kvist U (2009) Human sperm chromatin stabilization: a proposed model including zinc bridges. Mol Hum Reprod 16(1): 23-29.
- Björndahl L, Kvist U (1990) Influence of seminal vesicular fluid on the zinc content of human sperm chromatin. Int J Androl 13(3): 232-237.
- Kvist U, Björndahl L (1985) Zinc preserves an inherent capacity for human sperm chromatin decondensation. Acta Physiol Scand 124(2): 195-200.
- Kvist U (1980) Sperm nuclear chromatin decondensation ability. An in vitro study on ejaculated human spermatozoa. Acta Physiol Scand 486: 1-24.
- Roomans GM, Lundevall E, Björndahl L, Kvist U (1982) Removal of zinc from subcellular regions of human spermatozoa by EDTA treatment studied by X-ray microanalysis. Int J Androl 5(5): 478-486.
- Björndahl L, Kvist U (1985) Loss of an intrinsic capacity for human sperm chromatin decondensation. Acta Physiol Scand 124(2): 189-194.
- Björndahl L, Kjellberg S, Kvist U (1991) Ejaculatory sequence in men with low sperm chromatin-zinc. Int J Androl 14(3): 174-178.
- Seah LH, Wee BH (2015) Separation patterns of semen mixtures: The major-minor impact. Forensic Science International: Genetics Supplement Series 5: 582-583.
- Seah LH, Wee BH, Phoon YK (2018) A remodel of sperm chromatin stability corrects an interpretation fallacy. J Forensic Sci & Criminal Invest 10(2): 555782.






























