What Does the Medical Oncologist Expect from the Pathologist in Order to Treat a Metastatic Non-Small Cell Lung Cancer?
Maroun Sadek1 and Georges El Hachem1,2*
1Department of Medical Oncology, Institut Jules Bordet, Belgium
2Department of Medical Oncology, Erasme Hospital University Medical Center, Belgium
Submission: July 16, 2018; Published: August 27, 2018
*Corresponding author: Georges El Hachem, Department of Medical Oncology, Institut Jules Bordet & Erasme Hospital University Medical Center, Brussels, Belgium, Tel: 0032485853628, Email: george.el.hashem@hotmail.com/georges.elhachem@bordet.be
How to cite this article: Sadek M, El Hachem G. What Does the Medical Oncologist Expect from the Pathologist in Order to Treat a Metastatic Non-Small Cell Lung Cancer?. Ann Rev Resear. 2018; 3(2): 555608. DOI: 10.19080/ARR.2018.03.555608
Abstract
In the last decade, researchers provided us with significant advances in our understanding of lung cancer biology and management. They were able to identify many key driver events in lung carcinogenesis, resulting in the discovery of new modalities of targeted therapies towards a personalized medicine. In the same perspective, many clinical trials, together with the immunotherapy are including the patients according to the tumor mutational status and the expression of certain receptors or ligands. Thus, the pathological diagnosis is of high clinical relevance. Moreover, the pathologist is becoming an integrated and essential member during the multi-disciplinary team discussions, enriching our oncologic knowledge with a more developed and structured classification of non-small cell lung cancer. Here, in this review, we will discuss and list what Medical Oncologists are expecting to find in a pathology report in order to adequately treat the patients suffering from a metastatic non-small cell lung cancer.
Keywords: Non-small cell lung cancer; Mutation; Translocation; Targeted therapy
Abbrevations: FISH: Fluorescence In Situ Hybridization; IHC: Immune Histochemistry; RT-PCR: Reverse Transcriptase-Polymerase Chain Reaction; NGS: Next Generation Sequencing; TNM: Tumor Nodes Metastasis; SCLC: Small Cell Lung Cancer; EGFR: Epidermal Growth Factor Receptor; ALK: Anaplastic Lymphoma Kinase; TKIs: Tyrosine Kinase Inhibitors; TTF1: Thyroid Transcription Factor 1; DDR2: Discoidin Domain Receptor Tyrosine Kinase; NTRK1: Neurotrophic Receptor Tyrosine Kinase 1; MET: Mesenchymal Epithelial Transition; PDL1: Programmed Death Ligand 1; RET: Rearranged during Transfection
Introduction
Non-small cell lung cancers (NSCLCs) account for 85%-90% of lung cancers, while small cell lung cancers (SCLCs) are relatively decreasing in frequency in many countries over the past two decades [1]. The changes in the tumor-nodes-metastasis (TNM) classification headed towards a major change in the management of the NSCLC. Besides these prognostic modifications and the creation of the ‘’oligometastatic disease’’ entity, the current therapeutic decision relies on the following pathologic assessment: the morphologic description and the immunohistochemistry to define the histologic subtype, in addition to the molecular mutation/translocation identification.
The discovery of these mutations was practice changing in the metastatic setting only. It drastically changed the prognosis, not only improving the survival, but also the quality of life. However, in the early operable stage and other locally advanced stages which are treated with surgery and concomitant chemo-radiation therapy respectively, the pathologist is mainly responsible to identify the histological subgroup, and to exclude a small cell lung cancer. Controversially, it may be important to have the status of different molecular analyses (wild vs mutated) and other receptors expression that will serve as tumor archive. Upon disease recurrence, the Medical Oncologist will have a tumor specific platform to make an adequate therapeutic decision, especially if the metastatic site is not accessible to get a new specimen.
Discussion
NSCLCs are classified into adenocarcinoma, squamous cell lung carcinoma and adenosquamous lung carcinoma. Adenocarcinoma is the most common histological sub-type of lung cancer in contemporary series, accounting for approximately one-half of lung cancer cases [2]. Squamous-cell carcinoma comprises 25–30% of all lung cancer cases [3]. The reported incidence of adenosquamous carcinoma ranges from 0.4-4% of bronchogenic carcinomas [4].
1. Morphology and immunohistochemistry
Adenocarcinoma consists of four subtypes: acinar, papillary, adenocarcinoma in situ, and solid with mucous formation. They consist of glandular tumor cells producing mucous, and they grow in the peripheral part of the lung. They usually stain positive for thyroid transcription factor 1 (TTF1), cytokeratin 7 (CK7) and are negative for cytokeratin 20 (CK20) [5]. Controversially, squamous cell carcinoma consists of cells producing keratin in the same way as normal squamous epithelial cells. Histochemically, they tend to be TTF-1 negative, but positive for CK 5, CK 6 and P63 or P40. Through the tumor genesis, it was demonstrated that they develop from a pre-cancerous lesion as following: hyperplasia, metaplasia, dysplasia then carcinoma. These tumors generally grow in the central part of the lung in close relation to the large bronchi [6].
2. Targeted mutations and translocations
The identification of mutations in the epidermal growth factor receptor (EGFR) or rearrangements of the anaplastic lymphoma kinase (ALK) gene or proto-oncogene tyrosine-protein kinase ROS1 gene have led to a paradigm shift in the treatment of lung cancer by developing specific molecular therapies.
In NSCLC, the analysis of epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) inversions/ translocations are prerequisites for determining the appropriate tyrosine kinase inhibitor to be used as a targeted treatment in order to improve patient outcomes and survival [7,8].
2.1 EFGR testing
EGFR mutations are found in around 10%–12% of Caucasians with adenocarcinoma and are more frequent in never smokers, females and in patients of East Asian ethnicity. EGFR mutation testing is recommended in all patients with advanced nonsquamous cell carcinoma (NSCC) [9]. Molecular EGRF testing is not recommended in patients with an unequivocal diagnosis of squamous cell carcinoma, except in never/former light smokers [10].
EGFR mutations are found in around 10%–12% of Caucasians with adenocarcinoma and are more frequent in never smokers, females and in patients of East Asian ethnicity. EGFR mutation testing is recommended in all patients with advanced nonsquamous cell carcinoma (NSCC) [9]. Molecular EGRF testing is not recommended in patients with an unequivocal diagnosis of squamous cell carcinoma, except in never/former light smokers [10].
Real-time polymerase chain reaction (PCR), Sanger sequencing (paired with tumor enrichment), and next generation sequencing are the most commonly used methods to assess EGFR mutation status [14]. Of the known EGFR tyrosine kinase domain mutations, greater than 90% occur as short in-frame deletions in exon 19 or as point mutations in exon 21, the latter resulting in arginine replacing leucine at codon 858 (L858R). Two less common mutations occur at exons 18 and 21 [15].
The predictive effects of the drug-sensitive EGFR mutations, exon 19 deletion (LREA deletion mutant) and L858R, are well defined. Patients with these mutations have a significantly better response to erlotinib, gefitinib, or afatinib [16]. EGFR T790M is a mutation associated with acquired resistance to EGFR TKI therapy and has been reported in about 60% of patients with disease progression after initial response to erlotinib, gefitinib, or afatinib [17-19].
2.2 ALK translocation
ALK fusion is also encountered, but not exclusively, in never smokers, adenocarcinoma subtype and in younger patients, with a prevalence of around 5% in adenocarcinomas [20,21]. Inversion of the short arm of chromosome 2 leads to the joining of exons 1 to 13 of EML4 and exons 20 to 29 of ALK, resulting in the EML4–ALK chimeric protein, which is known to occur in approximately 4-7% of NSCLC [7].
Several methods for detecting ALK gene rearrangements are available, including fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), reverse transcriptase-polymerase chain reaction (RT-PCR) and next generation sequencing (NGS). There are two different RT-PCR approaches for ALK testing. One technique uses probes for fusion genes (ALK and EML4/KIF5B/ HIP133) [22,23], while the other compares different levels of amplification of small PCR products (50 and 30 portions of ALK transcripts) on the ALK gene (fusion partner independent) [24,25]
Rapid prescreening with IHC to assess for ALK rearrangements can be done; if positive, FISH analysis can confirm ALK positivity [26-28]. Therefore, highly effective ALKTyrosine kinase inhibitors (-TKIs) have been developed, with crizotinib being the first approved agent by both FDA and EMA for the treatment of ALK-rearranged, advanced NSCLC [29].
2.3 ROS1 translocation
ROS1 is an insulin receptor family tyrosine kinase. Its most common translocation aberration is with CD74 and occurs in 1-2% of patients with NSCLC [30]. Aberrant ROS1 kinase activity leads to downstream signaling of the PI3K and MAPK (phosphoinositide 3-kinase and mitogen-activated protein kinase) pathways [31]. NGS can also be used to assess whether ROS1 rearrangements are present, if the platform has been appropriately designed and validated to detect ROS1 rearrangements [32].
Treatment with crizotinib is FDA-approved and recommended for patients with the ROS1 translocation, including those who have received chemotherapy and those who are treatment-naïve [31]. Other TKIs like ceritinib and alectinib are also approved and associated with different response rate and toxicity profiles.
2.4 KRAS mutation
Kirsten rat sarcoma viral oncogene, or v-Ki-ras-2 (KRAS) mutations are present in approximately 30% of pulmonary adenocarcinomas and 5% of pulmonary squamous cell carcinomas [33]. KRAS mutations are also predictive of lack of benefit from EGFR TKI therapy [34-36]. Furthermore, KRAS mutation is mutually exclusive with EGFR mutation and ALK or ROS1 translocation. Once it is identified, there is no further need to test for the other druggable mutations. Nonetheless, there are no available targeted therapy for this mutation.
2.5 BRAF mutation
BRAF (v-Raf murine sarcoma viral oncogene homolog B) is a serine/threonine kinase that is part of the MAP/ERK signaling pathway. BRAF V600E is the most common mutation among the BRAF point mutations; it occurs in 1% to 2% of patients with lung adenocarcinoma [37]. Patients with BRAF V600E mutations are typically current or former smokers in contrast to those with EGFR mutations or ALK rearrangements who are typically non-smokers [38]. Mutations in BRAF do not overlap with EGFR mutations or ALK rearrangements [37,38]. Real-time PCR, Sanger sequencing, and NGS are the most commonly used methods to assess for BRAF mutations.
2.6 HER2
Case series or early-phase clinical trials suggest that patients with tumors harboring HER2 mutations often respond to trastuzumab and chemotherapy, to afatinib (being an EGFR/ HER2 Tyrosine Kinase inhibitor) or to ado-trastuzumab [39]. Other HER2-targeted agents are still under evaluation.
2.7 Other mutations
These are mutations described and reported in NSCLC. Unfortunately, we lack an approved TKI or standard targeted therapy outside a clinical trial when these mutations are present
2.7.1 PIK3CA
Mutations in Phosphatidylinositol-4, 5-Bisphosphate 3-Kinase Catalytic Subunit Alpha are seen in approximately 5% of squamous cell carcinomas of the lung, and numerous agents are under active investigation, with promising response rates at the expense of serious toxicities [40].
2.7.2 DDR2
Discoidin domain receptor tyrosine kinase 2- DDR2 mutations occur in approximately 4% of squamous cell NSCLC. DDR2 is a receptor tyrosine kinase that binds to collagen and promotes cellular proliferation [41].
2.7.3 NTRK1
Neurotrophic receptor tyrosine kinase 1- NTRK1 translocation occurs in <1% of NSCLC and include rearrangements in NTRK1, NTRK2, and NTRK3. NTRK activation leads to downstream signaling through the MAPK and PI3K pathways [42].
2.7.4 MET
Mesenchymal epithelial transition factor receptor -MET is a receptor tyrosine kinase that has a major function in tumor progression when bound to its ligand hepatocyte growth factor (HGF). MET mutations occur in 3% to 4% of NSCLC adenocarcinomas, 2% of squamous cell carcinomas, and 1% to 8% of other subtypes of lung cancer [43,44]. Several agents in preclinical stages are aiming to target the MET exon skipping mutations.
2.7.5 RET
The ’’Rearranged during Transfection’’-RET fusions are present in 1-2% of NSCLC/Adenocarcinoma of patients of both Asian and European descent. Several studies indicate that RET fusion occurs preferentially in young, never‐smoker, and lightsmoker patients [45]. RET fusions are also promising targets for personalized therapy in lung cancer.
2.8 Programmed Death Ligand 1 (PDL1)
The programmed death 1 (PD-1) receptor is an inhibitory T-cell receptor that is engaged by its two known ligands, PD-L1 (also known as B7-H1 or CD274) and PD-L2 (also known as B7- DC or CD273), primarily within the tumor microenvironment. It plays a crucial role in tumor immune escape.
Four different programmed death ligand 1 immunohistochemical assays are currently approved or in development as companion or complementary diagnostics to different immunotherapeutic agents in lung cancer: the Ventana SP142, the Ventana SP263 assay, the Dako 22C3 and the Dako 28-8 assay. Single agent pembrolizumab is approved as first-line therapy for patients with advanced NSCLC with PDL1 expression levels of 50% or more based on a phase 3 randomized trial (KEYNOTE-024) comparing pembrolizumab versus platinum-based chemotherapy [46]. Nivolumab is approved as a subsequent therapy for patients with metastatic NSCLC who have progressed on or after first line chemotherapy based on data from two phase 3 randomized trials (CheckMate-057, CheckMate-017). In the same perspective, based on the Keynote-010, pembrolizumab was approved as a second line treatment of metastatic NSCLC expressing PDL1 in >1% of the tumor cells [47-50]. The regimen consisting of atezolizumab/carboplatin/paclitaxel/bevacizumab is approved as first line therapy for patients with metastatic nonsquamous NSCLC based on a recent phase 3 randomized trial (IMpower150) [51].
Conclusion
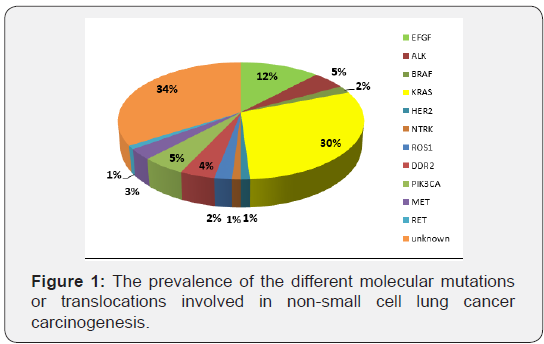
To conclude, the outcome of the patients with metastatic NSCLC has drastically changed with an improved overall survival without affecting the quality of life. Through the knowledge of lung cancer carcinogenesis, the pathologic analysis of the biopsies is no more only dependent on morphological and immunohistochemical features; it is mandatory to thoroughly analyze the molecular profiles and the signaling pathways because many targeted therapies are available. Furthermore, in the era of immunotherapy, new challenges are asked from our pathologist colleagues: the PDL1 expression, the tumor mutational burden and other receptors or protein expressions under investigation. Consequently, new horizons to precision medicine and subsequently to newer therapeutic strategies are currently opened, leading to new toxicity profiles: immunologic, broad spectrum of serious adverse events and mainly an emerging challenging financial toxicity (Figure 1).
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61(2): 69-90.
- Janssen-Heijnen ML, Coebergh JW, Klinkhamer PJ, Schipper RM, Splinter TA, et al. (2001) Is there a common etiology for the rising incidence of and decreasing survival with adenocarcinoma of the lung? Epidemiology 12(2): 256-258.
- Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, Colditz GA et al. (2008) Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control 17(3): 198-204.
- Filosso PL, Ruffini E, Asioli S, Giobbe R, Macri L, et al. (2011) Adenosquamous lung carcinomas: a histologic subtype with poor prognosis. Lung Cancer 74(1): 25-29.
- Stenhouse G, Fyfe N, King G, Chapman A, Kerr KM (2004) Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol 57(4): 383-387.
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, et al. (2011) International association for the study of lung cancer/ american thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6(2): 244-285.
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, et al. (2007) Identification of the transforming EML4-ALK fusion gene in non-smallcell lung cancer. Nature 448(7153): 561-566.
- Thunnissen E, Bubendorf L, Dietel M, Elmberger G, Kerr K, et al. (2012) EML4-ALK testing in non-small cell carcinomas of the lung: a review with recommendations. Virchows Arch 461(3): 245-257.
- Kerr KM, Bubendorf L, Edelman MJ, Marchetti A, Mok T, et al. (2014) Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol 25(9): 1681-1690.
- Rekhtman N, Paik PK, Arcila ME, Tafe LJ, Oxnard GR, et al. (2012) Clarifying the spectrum of driver oncogene mutations in biomarkerverified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res 18(4): 1167-1176.
- Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, et al. (2008) The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr Protoc Hum Genet Chapter 10: Unit 10-11.
- Sholl LM, Cagle PT, Lindeman NI, et al. (2016) Template for reporting results of biomarker testing of specimens from patients with non-small cell carcinoma of the lung. Version: Lung Biomarkers 1.3.0.0, College of American Pathologists, USA
- Sholl LM, Xiao Y, Joshi V, Yeap BY, Cioffredi LA, et al. (2010) EGFR mutation is a better predictor of response to tyrosine kinase inhibitors in non-small cell lung carcinoma than FISH, CISH, and immunohistochemistry. Am J Clin Pathol 133(6): 922-934.
- Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, et al. (2013) Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 8(7): 823-859.
- Ladanyi M, Pao W (2008) Lung adenocarcinoma: guiding EGFRtargeted therapy and beyond. Mod Pathol 21 Suppl 2: S16-S22.
- Langer CJ (2013) Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol 31(27): 3303-3306.
- Yu PP, Vose JM, Hayes DF (2015) Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol 33(31): 3533- 3534.
- Riely GJ, Yu HA (2015) EGFR: the paradigm of an oncogene-driven lung cancer. Clin Cancer Res 21(10): 2221-2226.
- Onitsuka T, Uramoto H, Nose N, Takenoyama M, Hanagiri T, et al. (2010) Acquired resistance to gefitinib: the contribution of mechanisms other than the T790M, MET, and HGF status. Lung Cancer 68(2): 198-203.
- Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, et al. (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363(18): 1693-1703.
- Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, et al. (2009) Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 27(26): 4247-4253.
- Fu S, Wang F, Shao Q, Zhang X, Duan LP, et al. (2015) Detection of EML4-ALK fusion gene in Chinese non-small cell lung cancer by using a sensitive quantitative real-time reverse transcriptase PCR technique. Diagn Mol Pathol 23(4): 245-254.
- Zhang NN, Liu YT, Ma L, Wang L, Hao XZ, et al. (2014) The molecular detection and clinical significance of ALK rearrangement in selected advanced non-small cell lung cancer: ALK expression provides insights into ALK targeted therapy. PLoS One 9(1): e84501.
- Pan Y, Zhang Y, Li Y, Hu H, Wang L, et al. (2014) ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion patternspecific clinicopathologic, histologic and cytologic features. Lung Cancer 84(2): 121-126.
- Wang R, Pan Y, Li C, Hu H, Zhang Y, et al. (2012) The use of quantitative real-time reverse transcriptase PCR for 50 and 30 portions of ALK transcripts to detect ALK rearrangements in lung cancers. Clin Cancer Res 18(17): 4725-4732.
- Ali G, Proietti A, Pelliccioni S, Niccoli C, Lupi C, et al. (2014) ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med 138(11): 1449- 1458.
- Rogers TM, Russell PA, Wright G, Wainer Z, Pang JM, et al. (2015) Comparison of methods in the detection of ALK and ROS1 rearrangements in lung cancer. J Thorac Oncol 10(4): 611-618.
- Mino-Kenudson M, Chirieac LR, Law K, Hornick JL, Lindeman N, et al. (2010) A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res 16(5): 1561-1571.
- Iacono D, Chiari R, Metro G, Bennati C, Bellezza G, et al. (2015) Future options for ALK-positive non-small cell lung cancer. Lung Cancer 87(3): 211-219.
- Acquaviva J, Wong R, Charest A (2009) The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta 1795(1): 37-52.
- Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, et al. (2012) ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 30(8): 863-870.
- Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, et al. (2014) Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371(21): 1963-1971.
- Slebos RJ, Kibbelaar RE, Dalesio O, Kooistra A, Stam J, et al. (1990) K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med 323(9): 561-565.
- Miller VA, Riely GJ, Zakowski MF, Li AR, Patel JD, et al. (2008) Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol 26(9): 1472-1478.
- Roberts PJ, Stinchcombe TE (2013) KRAS mutation: should we test for it, and does it matter? J Clin Oncol 31(8): 1112-1121.
- Tsao MS, Aviel-Ronen S, Ding K, Lau D, Liu N, et al. (2007) Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non-small-cell lung cancer. J Clin Oncol 25(33): 5240-5247.
- Planchard D, Besse B, Groen HJ, Souquet PJ, Quoix E, et al. (2016) Dabrafenib plus trametinib in patients with previously treated BRAF (V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 17(7): 984-993.
- Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, et al. (2011) Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 29(15): 2046-2051.
- De Grève J, Teugels E, Geers C, Decoster L, Galdermans D, et al. (2012) Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 76(1): 123-127.
- Kawano O, Sasaki H, Endo K, Suzuki E, Haneda H, et al. (2006) PIK3CA mutation statusin Japanese lung cancer patients. Lung Cancer 54(2): 209-215.
- Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, et al. (2011) Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov 1(1): 78-89.
- Farago AF, Le LP, Zheng Z, Muzikansky A, Drilon A, et al. (2015) Durable clin-ical response to entrectinib in NTRK1-rearranged non-small cell lung cancer. J Thorac Oncol 10(12):1670-1674.
- Frampton GM, Ali SM, Rosenzweig M, Chmielecki J, Lu X, et al. (2015) Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 5(8): 850-859.
- Cancer Genome Atlas Research Network (2014) Comprehensive molecular pro- filing of lung adenocarcinoma. Nature 511(7511): 543- 550.
- Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, et al. (2012) RET, ROS1 and ALK fusions in lung cancer. Nat Med 18(3): 378-381.
- Reck M, Rodriguez Abreu D, Robinson AG, Hui R, Csőszi T, et al. (2016) Pembrolizumab versus chemotherapy for PDL1-positive non-small cell lung cancer. N Engl J Med 375: 1823-1833.
- Borghaei H, Paz Ares L, Horn L, Spigel DR, Steins M, et al. (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small cell lung cancer. N Engl J Med 373(17): 1627-1623.
- Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WEE, et al. (2015) Nivolumab versus docetaxel in advanced squamous cell non-small cell lung cancer. N Engl J Med 373: 123-135.
- Melosky B, Chu Q, Juergens R, Leighl N, McLeod D, et al. (2016) Pointed progress in second line advanced non-small cell lung cancer: the rapidly evolving field of checkpoint inhibition. J Clin Oncol 34(14): 1676-1688.
- Norum J, Antonsen MA, Tollåli T, Al-Shibli K, Andersen G, et al. (2017) Pembrolizumab as second-line therapy in non-small cell lung cancer in northern Norway: budget impact and expected gain--a model-based analysis. ESMO Open 2(3): e000222.
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. (2018) Atezolizumab for First line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24): 2288-2301.






























