Protein – Protein Interactions and Protein Folding as Therapeutic Targets
Eswari Beeram*
Department of Biochemistry, Sri Venkateswara University, India
Submission: January 17, 2018; Published: February 06, 2018
*Corresponding author: Eswari beeram, Department of Biochemistry, Sri Venkateswara University, Tirupati-517502, Andhra Pradesh, India, Email: eshu.sonu@gmail.com
How to cite this article: Eswari Beeram. Protein – Protein Interactions and Protein Folding as Therapeutic Targets. Ann Rev Resear. 2018; 1(2): 555560. DOI: 10.19080/ARR.2018.01.555560
Abstract
The process of protein folding to native protein was one of the most peculiar processes which is ravelled by many of the anti microbial agents and genetically altered cells and getting successful by introducing loss of function or gain of function mutations related to the process. Cystic fibrosis is one of the well understood diseases that are discussed here. Unfolded proteins are guided by molecular chaperones folding them in to its native state. Whereas some of the mutations lead to the generation of misfolded proteins whose study is difficult as aggregation properties in the cell differ by type of fusion proteins used .So, continuous effort is being made in this area and the results are promisive but, as most of the proteins contain un organised regions of 30 amino acids which is necessary for non specific interactions with proteins. So, as protein -protein interactions play a major role, designing therapeutic drugs pertaining to protein -protein interactions is found to be useful.
Introduction
Proteins as known are the workloads of the cell. So they should be maintained as proper by without loss of functional activity in the cell. Protein folding is maintained by the cellular chaperones. In unfolded state the free energy change and entropy was found to be very high which can be reduced by folding in to native state. Some of the proteins with mutations are mainly responsible for several misfolding disorders like Alzheimer's disease which forms oligomers that are highly toxic to the cell.
The major thing to be considered in protein folding is that the native protein should contain minimum in its Gibbs free energy [1]. In folding there is formation of large intermediates in between the unfolded and the native proteins. Mostly proteins show monophasic unfolding process and multi phasic refolding kinetics. Based on NMR and circular dichroism the proteins have several intermediates in the folding process [1].
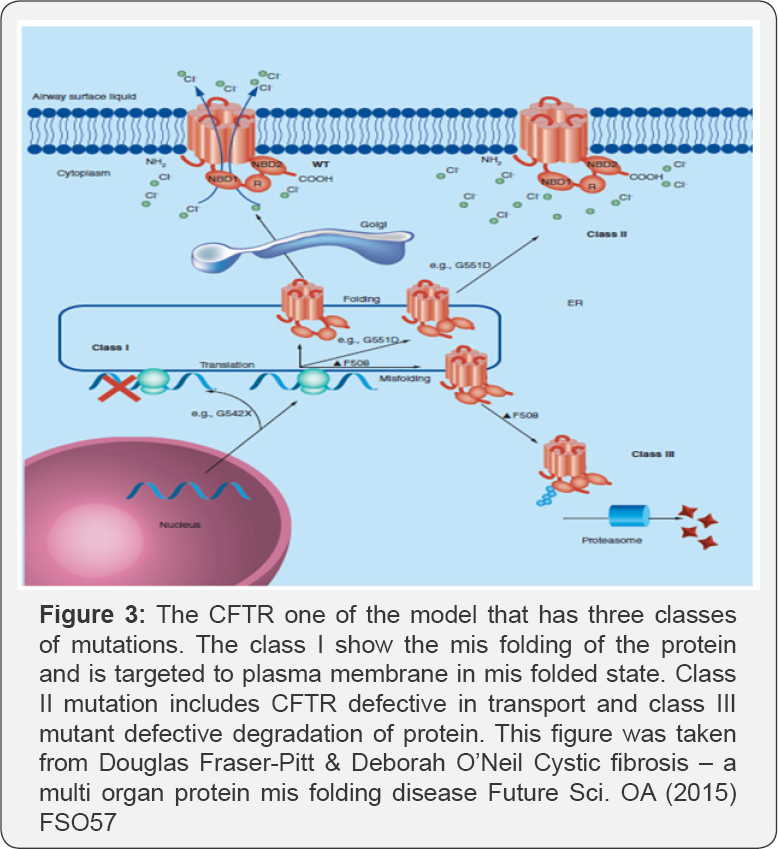
Protein folding is a highly ordered process. The individual domains of the protein associate autonomously to form the native state. In native form the c- terminal and N-terminal regions are adjacent to each other in which the substrate binding ability was attributed to the C-terminal end [2]. But however in some proteins just combining the domains doesn't give the native state indicating that long range interactions are needed for a protein to attain its native state. There are also various sub domains in the protein which also associate autonomously to form the native form. The protein misfolding mainly involves exposure of the buried hydrophobic residues to the solvents and formation of the toxic aggregates in the cell. They should be reordered again in order to prevent the exposure of hydrophobic residues to the environment. Protein misfolding is sensed by some of the chaperones and folds them in proper way and prevents the protein to form toxic oligomers in the cell [3]. Proteostasis which makes the cells to respond to the stress and causes chaperones to assist the proteins to fold in to its native state. Cystic fibrosis which was mainly due to non function of a protein CFTR which is a water and surface rehydration ion channel necessary for maintaining the moisture in the air ways whose dysfunction leads to formation and accumulation of the thick mucus leading to inflammation and the disease Cystic fibrosis.
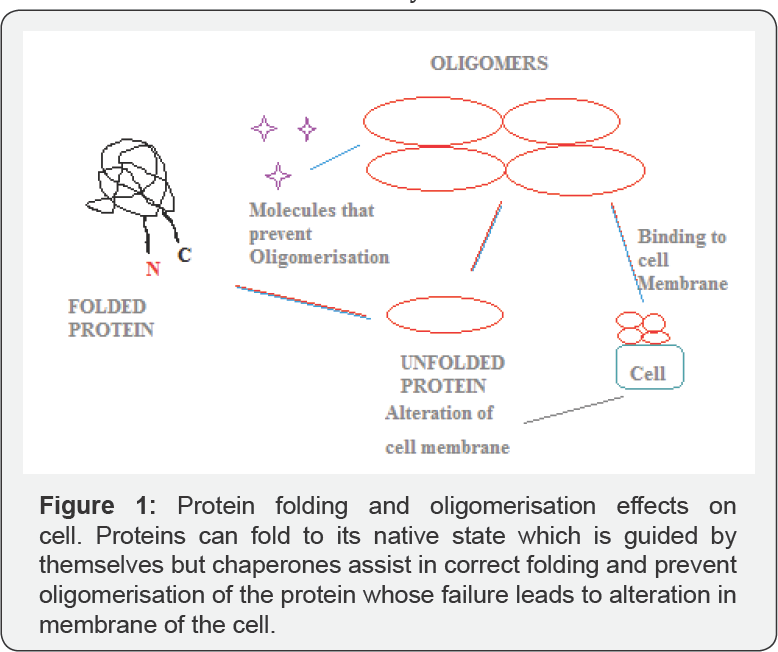
Another protein misfolding disease is the alzeihmers disease in which the protein aggregates and form toxic oligomers which bind to the cell surface receptors leading to the alterations of the cell membrane [4]. Seminal amyloidal protein was the protein that binds to the toxic oligomers and serves as a model for studying the disease [5-7]. This review mainly focuses on the protein misfolding and its therapeutic application on the cell (Figure 1).
Drugs that Selectively Inhibit Protein Folding
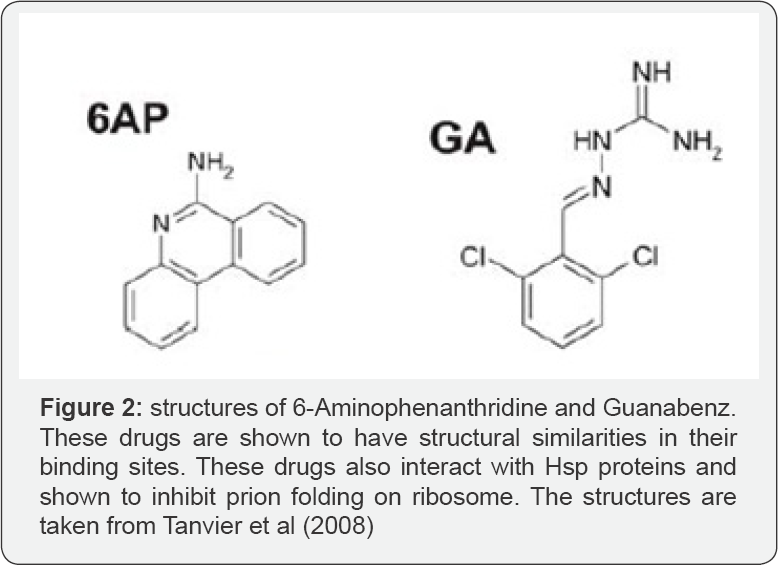
As per Tanvier et al. [8] 6-Aminophenanthridine (6AP) and Guanabenz (GA the drug used for the treatment of hypertension) drugs inhibit protein folding on domain V of ribosome by interacting with larger subunit of eukaryotes as well as prokaryotes without effecting the protein synthesis on ribosome. They are known to interact in a Trans manner affecting the various ribosomal proteins especially in yeast by 6AP indicating its specificity against eukaryotes than that of GA (Figure 2). From the studies of Yanhong Pang et al. [9] 6AP inhibits the protein folding in the ribosome through competition.
Protection from the Picorna Virus by Preventing the Protein Folding
Hsp 90 is one of the molecular chaperones necessary for protein folding. So, inhibitors of Hsp 90 were proven as potential targets of viral replication. From the previous studies of Ron Geller et al. [10] Geldanamycin was proven to be one of the drug that inhibit Hsp 90. The inhibitor mainly prevents the interaction of Hsp 90 with its co-chaperone P23 so; preventing the folding of capsid protein of virus P1 thereby preventing the assembly of virus and its replication [11-14]. In reticulocytes where the proteo some is inhibited by hemin proved that the capsid will be in the unfolded state in the absence of Hsp90 [15].
Protein Folding Aided First Through non Specific Interactions
Recent advance in science about studies of CREB protein by Eric Sauter et al. [16] detailed that the phosphory lating kinase domain of the CREB protein binds non specifically with KIX domain of same protein and aids in folding of the protein through non specific interaction until it reaches the target.So, targeting the proteins by drugs that disrupt the protein - protein interactions are a widely studied aspect to develop new therapeutic and pharmacological targets now (Figure 3).
Formation of Aggregates Depends on Sequence of the Proteins
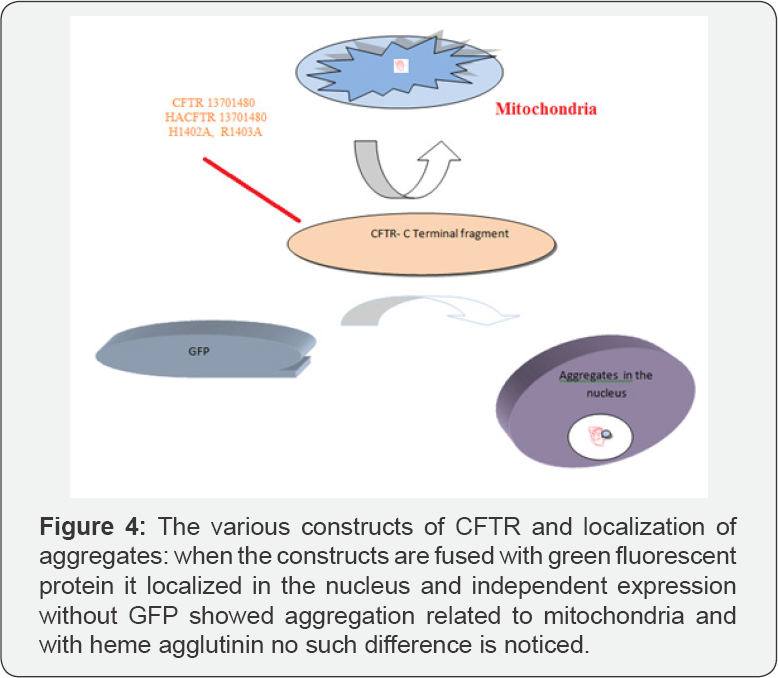
Aggregation of proteins is the major problem in most of the protein misfolding disorders. One of the diseases that are associated with aggregation is cystic fibrosis. So, understanding the mechanism of aggregation formation and its distribution with respect to the cell is very useful. From the previous studies of [17,18], the CFTR C-terminal fragment showed distinct distribution with perinuclear accumulation and without GFP showed aggregation of protein in mitochondria and the amino acid substitutions changed the aggregation pattern of protein [19]. Huntington disease and CFTR showed distinct distribution of aggregates. More overly Hsp 90, Hsp 40, Hsp 70was known to be associated with the aggregates and the unknown motor protein of microtubules is necessary for movement of the protein aggregates to the periphery of the cell [20] (Figure 4).
Discussion
Protein folding is one of the complex process in the cell which should be regulated, as accumulation of misfolded proteins are the major cause of most of the diseases like alzeihmers disease, Huntington's disease and cystic fibrosis. In all cases the misfolded proteins are aggregated inside the cell and lead to strong immunogenic reactions causing the death of the cell. As in other case misfolding mutations in proteins of genetically altered cells like that of myc-c is one of the major problems identified now. Recent advances have shown that the protein - protein interactions like that of CREB protein and most other proteins has been used as one of the powerful mode for developing the therapeutic targets against the viruses and cancer. Prevention of aggregation of fibrils intracellular is one of the possible modes to prolong the life expectancy of an alzeihmers disease individual. Inhibitors of chaperones should be considered again as it also inhibits the folding of host protein also although it occurs autonomously.
References
- JM Yon (2001) Protein folding: a perspective for biology, medicine and biotechnology. Braz J Med Biol Res 34(4): 419-435.
- Rajiah Aldrin Denny, Lori Krim Gavrin, Eddine Saiah (2013) Recent developments in targeting protein misfolding diseases. Bioorganic & Medicinal Chemistry Letters 23: 1935-1944.
- Narayan P, Orte A, Clarke RW, Bolognesi B, Hook S, et al. (2012) The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-p(1-40) peptide. Nat Struct Mol Biol 19(1): 79-83.
- Campioni S, Mannini B, Zampagni M, Pensalfini A, Parrini C, et al. (2010) A causative link between the structure of aberrant protein oligomers and their toxicity. Nat Chem Biol 6(2): 140-147.
- Pepys MB, Booth DR, Hutchinson WL, Gallimore JR, Collins PM, et al. (1997) Amyloid P component. A critical review. Int J Exp Clin Invest 4: 274-295.
- Hawkins PN, Pepys MB (1995) Imaging amyloidosis with radiolabelled SAP. Eur J Nucl Med 22(7): 595-599.
- Jager PL, Hazenberg BP, Franssen EJ, Limburg PC, van Rijswijk (1998) Kinetic studies with iodine-123-labeled serum amyloid P component in patients with systemic AA and AL amyloidosis and assessment of clinical value. Journal of Nuclear Medicine 39(4): 699-706.
- Deborah Tribouillard Tanvier, Suzana Dos Reis, Fabienne Gug, Cecile Voisset, Vincent Beringue, et al. (2008) Protein Folding Activity of Ribosomal RNA Is a Selective Target of Two Unrelated Antiprion Drugs. PLoS ONE 3(5): 2174.
- Yanhong Pang, Sriram Kurella, Cecile Voisset, Dibyendu Samanta, Debapriya Banerjee, et al. (2013) The Antiprion Compound 6-Aminophenanthridine Inhibits the Protein Folding Activity of the Ribosome by Direct Competition J Biol Chem 288(26): 19081-19089.
- Ron Geller, Marco Vignuzzi, Raul Andino, Judith Frydman (2007) Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev 21(2): 195-205.
- Young JC, Moarefi I, Hartl FU (2001) Hsp90: A specialized but essential protein-folding tool. J Cell Biol 154(2): 267-273.
- Picard D (2002) Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci 59: 1640-1648.
- Wegele H, Muller L, Buchner J (2004) Hsp70 and Hsp90-A relay team for protein folding. Reviews of Physiology, Biochemistry and Pharmacology 151: 1-44.
- Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, et al. (2006) Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 440(7087): 1013-1017.
- Haas AL, Rose IA (1981) Hemin inhibits ATP-dependent ubiquitin- dependent proteolysis: Role of hemin in regulating ubiquitin conjugate degradation. Proc Natl Acad Sci USA 78(11): 6845-6848.
- Eric Sauter (2007) Study Reveals Process Linking Disordered Protein Folding and Binding 7(18).
- Milewski MI, Mickle JE, Forrest JK, Stafford DM, Moyer BD (2001) A PDZ-binding motif is essential but not sufficient to localize the C terminus of CFTR to the apical membrane. Journal of Cell Science 114: 719-726.
- Michal I Milewski, John E Mickle, John K Forrest, Bruce A Stanton, Garry R Cutting (2002) Aggregation of misfolded proteins can be a selective process dependent up on peptide composition.
- Johnston JA, Ward CL, Kopito RR (1998) Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143(7): 1883-1898.
- Hogle JM (2002) Poliovirus cell entry: Common structural themes in viral cell entry pathways. Annu Rev Micro biol 56: 677-702.
- Douglas Fraser Pitt, Deborah O Neil (2015) Cystic fibrosis-a multiorgan protein misfolding disease. Future Sci OA 1(2): FSO57






























