Advances in the Prevention and Treatment of Necrotizing Enterocolitis: A New Era in Neonatal Care
Miguel Eduardo Rodriguez Rodriguez1, Akshit Bhambri2, Ileana Patricia Crespin Henriquez3, Rishita Dave4, Linda Boahemaah-Kontoh5, Jeenesh Chandra Shrestha6, Jesus Alejandro Cordova Guilarte7, Kinjal Shah8, Praise Abiola Idowu9 and Maria Isabel Gomez-Coral10*
1Universidad de Oriente, Venezuela. Larkin Community Hospital, Miami, Florida, USA
2Esic Medical College, Hyderabad, India
3Universidad Evangélica de El Salvador, El Salvador
4University of Medicine and Health Sciences, Saint Kitts
5Vinnitsa National Medical University, Ukraine
6Nepal Medical College, Nepal
7Universidad de Oriente, Venezuela
8Rutgers Edwards J. Bloustein School of Planning and Public Policy, NJ, USA
9Richmond Gabriel University, Saint Vincent and the Grenadines
10Universidad del Valle, México
Submission: October 04, 2024; Published: October 15, 2024
*Corresponding author: Maria Isabel Gomez-Coral, Universidad del Valle, México
How to cite this article: Miguel Eduardo Rodriguez Rodriguez, Akshit Bhambri, Ileana Patricia Crespin Henriquez, Rishita Dave, Linda Boahemaah-Kontoh, et al. Advances in the Prevention and Treatment of Necrotizing Enterocolitis: A New Era in Neonatal Care. Adv Res Gastroentero Hepatol, 2024; 21(1): 556051.DOI: 10.19080/ARGH.2024.21.556051.
Abstract
Necrotizing enterocolitis (NEC), a severe gastrointestinal illness primarily occurring in premature infants, causes inflammation and tissue death in the intestines. NICUs continue to see a high percentage of deaths from this condition, particularly among infants born with very low birth weights. Several factors, including an underdeveloped immune system and disruptions in the gut microbiota, cause NEC. A recent development in neonatal care has focused on early detection through biomarkers and advanced imaging techniques, improving the ability to identify and intervene earlier in high-risk infants. A number of preventive measures are being explored to lower the risk, including breastfeeding, probiotics, and experimental techniques such as fecal microbiota transplants. The development of new therapies, such as stem cells and targeted biological treatments, aims to reduce inflammation and promote intestinal regeneration. While minimally invasive surgical techniques like laparoscopy can also help manage severe cases, personalized medicine is beginning to play a role in tailoring treatment plans to each infant’s specific risk factors. As a result of these ongoing innovations, it is believed that NEC’s overall impact will be reduced, and the outcomes for affected infants will be improved.
Keywords: Necrotizing Enterocolitis (NEC); Neonatal Gastrointestinal Disorders; Preventive and Therapeutic Innovations
Abbreviations: NEC: Necrotizing Enterocolitis; NICU: Neonatal Intensive Care Unit; NPO: Nil Per Os (nothing by mouth); NIRS: Near-Infrared Spectroscopy; AI: Artificial Intelligence; MSCs: Mesenchymal Stem Cells; EPO: Erythropoietin; EGF: Epidermal Growth Factor; SBS: Short Bowel Syndrome; EN: Enteral Nutrition; PN: Parenteral Nutrition; IF: Intestinal Failure; PNALD: Parenteral Nutrition-Associated Liver Disease; MBD: Metabolic Bone Disease; SNPs: Single Nucleotide Polymorphisms
Introduction
Necrotizing enterocolitis (NEC) is a life-threatening gastrointestinal condition primarily affecting neonates, especially those born prematurely. It occurs in about 5% of infants admitted to neonatal intensive care units (NICUs), with a higher incidence of 9% in those born at 22 to 29 weeks of gestation. Several risk factors have been identified, with prematurity, low birth weight, and formula feeding being the most significant. Mortality rates range from 10% to 50%, but in the most severe cases, where perforation, peritonitis, and sepsis are present, mortality can approach 100%. Symptoms include poor feeding, vomiting, diarrhea, and progressive abdominal distension. Diagnosis is confirmed through abdominal radiographs, where findings such as dilated bowel loops, pneumatosis intestinalis, and portal venous gas are characteristic of NEC. The initial management includes immediate cessation of enteral feedings, placing the patient on nil per os (NPO), and nasogastric decompression of the dilated bowels. Broadspectrum intravenous antibiotics are started promptly. In cases of clinical deterioration, bowel perforation, or failure of medical management, surgical intervention is required. Laparotomy is the standard surgical approach, focusing on removing only necrotic or perforated sections of the bowel to preserve as much intestine as possible [1,2]. As current treatment strategies have limited success and have devastating consequences, new preventive and therapeutic measures are essential to addressing the high burden of necrotizing enterocolitis. A number of promising strategies are being developed for reducing the incidence and severity of NEC, including bioengineered tissues, personalized probiotic therapies, and targeted anti-inflammatory drugs. A new generation of early diagnostics, such as non-invasive biomarkers and advanced imaging techniques, may make it possible to detect cancer more quickly and accurately, preventing its progression to a more severe stage. As a result of these advances, the burden of the disease could be significantly reduced, survival rates could be improved, and the quality of life for affected infants could be enhanced in the long run [3-6].
Pathogenesis of NEC: Shifting Paradigms
NEC is a common intestinal disorder in which the lining of the intestine becomes inflamed, resulting in tissue death and potential sloughing. In most cases, this condition affects preterm or critically ill infants and typically develops while they are still in the hospital [7]. In preterm infants, NEC generally starts within the first 2 to 3 weeks of life, even in those who seem to be recovering well. As a result of the timing of the onset of symptoms and the underlying cause of NEC, neonatologists classify NEC into different types. A total of four types of NEC exist, classic, transfusion-associated, atypical, and term infants [8]. The most common form of NEC affects infants born before 28 weeks gestation and begins three to six weeks after birth. The condition usually manifests without warning in neonates who have been stable and progressing well prior to the onset of the condition [8]. Transfusion-associated NEC occurs in approximately one in three premature infants who receive a blood transfusion for anemia and may develop NEC within three days of the transfusion [8]. Atypical NEC is rare and some infants develop NEC within the first week of life or before their initial feeding [8]. Lastly, term infant NEC develops in fullterm babies who have an underlying birth defect. Potential causes include congenital heart conditions, gastroschisis (where the intestines forms outside the body), or low oxygen levels at birth [8].
NEC is one of the leading causes of illness and death in premature infants. Its pathophysiology is thought to result from an overactive innate immune response to the gut microbiota in the underdeveloped intestinal tract of preterm infants. The combined effects of intestinal inflammation, ischemia, and bacterial invasion result in an immature immune response that damages the tissues of the intestine [9]. Also, preterm infants have an underdeveloped epithelial barrier, which makes them more susceptible to bacterial colonization, consequently, harmful bacteria can invade the intestinal wall, causing inflammation [10]. Ischemia also damages tissues in the intestines, which further weakens the barrier and allows bacteria to enter the bloodstream [9-10]. A premature infant’s immune system is less capable of fighting bacterial infections as a result of exaggerated inflammation, it is believed that a combination of these factors contributes to the development of NEC [9].
Researchers have recently examined the role of intestinal microbiota, genetic predisposition, and inflammatory mediators in the pathogenesis of NEC. When an infant is premature, the gut microbiota undergoes significant changes, which contribute to a dysbiotic intestinal environment. It is common for NEC cases to exhibit dysbiosis, or a microbial imbalance, particularly due to an overgrowth of Proteobacteria [11]. As a result of these changes in gut bacteria, an exaggerated immune response is induced, causing inflammation and damage to the intestinal wall [12]. Genetic susceptibility also plays a role, with studies showing certain gene mutations that impair microbial sensing and immune responses, predisposing infants to NEC. Genome-wide association studies have identified mutations in pattern recognition receptors, critical for recognizing gut bacteria, that increase NEC susceptibility [12]. The immune response to bacterial invasion is aggravated by inflammatory mediators, including cytokines, which contribute to disease progression.
Early Detection: Biomarkers and Predictive Tools
Advances in preventing and treating NEC, particularly early detection, have improved neonatal care. Diagnosing NEC as early as possible is critical, and emerging biomarkers will improve the ability to predict the disease before it manifests clinically. Biomarkers such as cytokines, calprotectin, and intestinal fatty-acid binding protein (I-FABP) can provide insight into gut inflammation early. Neonatologists can use these biomarkers to identify infants at higher risk of developing NEC and intervene more timely and effectively [13,14]. Besides biomarker research, point-of-care innovations are revolutionizing how clinicians detect NEC early. Minimally invasive tools such as near-infrared spectroscopy (NIRS) and gut-specific ultrasound are being developed to monitor intestinal oxygenation and structure. NIRS can continuously monitor splanchnic oxygenation to identify infants with compromised intestinal perfusion before clinical symptoms appear.
In contrast, gut ultrasound can detect early pathophysiological changes associated with NEC through real-time intestinal motility, wall thickness, and perfusion imaging. By combining these tools, at-risk neonates can be identified earlier, improving their outcomes [14]. As a result of AI integration into neonatal care, new possibilities for predicting neonatal death exist. A machine learning and artificial intelligence model analyzes complex datasets, such as gestational age, birth weight, and early clinical signs, to predict which newborns are most likely to develop NEC; clinicians can use these models to sift through large volumes of clinical data and identify patterns they might not otherwise recognize. Artificial intelligence-driven tools are now being developed to supplement traditional diagnostic methods and facilitate earlier intervention [15].
Preventive Strategies: Moving Beyond Traditional Approaches
Several factors, including immaturity of the immune system, structural defects in the intestinal epithelial barrier, dysmotility of the gut, and impaired microvascular circulation, make premature newborns more likely to develop NEC. Regulation of the gut microbiome has decreased the chances of NEC by supporting these functions. As a result of the bactericidal and immunemodulating properties of breast milk, these substances promote the growth of a healthy gut microbiome and suppress the growth of pathogens known as Escherichia coli, Staphylococcus aureus, and Candida species. As a live microorganism, a probiotic encourages bifidobacteria and lactobacillus to grow in the gut, improving microbial balance. If breast milk is unavailable, prebiotics and probiotics can be added to infant formula to replicate some of its benefits [16-19]. It is becoming increasingly common to perform fecal microbiome transplants (FMT) and fecal filtrate transplants (FFT) to treat NEC; however, their application remains debated. During FMT, all contents, including bacteria and potential toxins, are transferred from a donor to a recipient, which increases the risk of sepsis and mortality.
Meanwhile, FFT uses micropore filtering or ultraviolet radiation to remove unwanted bacteria and toxins while preserving bacteriophages that may help prevent NEC. Despite showing promise in laboratory experiments with pigs and mice, FFT has not been studied on preterm infants due to clinical applications of this technology requiring strict safety protocols, extensive donor material screening, and validated methods to remove harmful components. It is necessary to conduct further research to evaluate FFT’s safety and effectiveness in preterm infants [20-21].
Novel Therapeutic Interventions
Despite advancements in therapeutic techniques, necrotizing enterocolitis persists as a critical condition in neonatal intensive care units, and by analyzing this data, innovative approaches that may enhance NEC outcomes are highlighted.
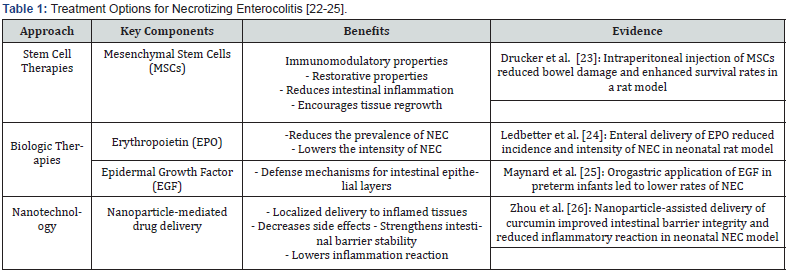
Stem Cell Therapies
In the treatment of NEC, mesenchymal stem cells (MSCs) have been considered a breakthrough. As a result of their immunoregulatory and regenerative properties, they are an effective means of reducing intestinal inflammation and promoting tissue regeneration. In a preclinical trial, Drucker et al. [23] demonstrated that intraperitoneal injection of MSCs significantly reduced bowel injury and increased survival rates in rats with NEC [22]. By using this technique, the inflammatory response appears to be adjusted, and the intestinal barrier appears to be strengthened.
Biologic Therapies
Targeted anti-inflammatory drugs and growth factors are being explored to reduce inflammation in NEC. Erythropoietin (EPO), established for promoting erythropoiesis, has shown effectiveness in NEC prevention and management. Ledbetter et al. [24] documented that enteral delivery of EPO decreased the occurrence and severity of NEC in a neonatal rat model [23]. The epidermal growth factor (EGF) has also been shown to have protective effects on intestinal epithelium; in a randomized clinical trial Maynard et al. [25] demonstrated that administering EGF via the orogastric route decreased the incidence of NEC on preterm infants [24].
Nanotechnology in NEC
Nanotechnology has enabled the delivery of precision drugs and the treatment of NEC; for example, nanoparticles can deliver drugs directly to inflamed intestinal areas, preventing damage to healthy cells and minimizing side effects. In a neonatal NEC model, Zhou et al. [26] designed a nanoparticle-mediated delivery platform for curcumin that improved intestinal barrier integrity and decreased inflammatory response [25]. These innovative approaches not only hold promise for NEC treatment but also have the potential to significantly advance neonatal healthcare. Table 1 provides an overview of the various treatment options for Necrotizing Enterocolitis, along with their respective benefits and evidence of their effectiveness.
Surgical Innovations in NEC Management
Minimally Invasive Surgery: Laparoscopic or roboticassisted surgery
Laparoscopic surgery has emerged as a promising alternative to traditional open laparotomy. Based on Smith & Thyoka’s [27] study, laparoscopy can be beneficial, particularly when surgical intervention is unclear. Helping avoid unnecessary laparotomies in select cases and reducing surgical trauma [26]. A retrospective cohort study by Montalva L [28], Incerti, F., Qoshe, L., et al. (2023) found that early laparoscopic-assisted surgery may reduce inflammation and postoperative complications in infants with necrotizing enterocolitis (NEC). However, to confirm these findings and to assess the long-term effects of laparoscopy, a prospective, randomized trial is required [27]. A case series by Leva E [29], Di Cesare, A., Canazza, L., et al. (2010) reported that gasless laparoscopy in 8 newborns with necrotizing enterocolitis (NEC) can be effective in detecting perforations and managing the condition, particularly in low birth weight infants [28]. According to Clark & Mackinlay [30], laparoscopy allows for excellent visualization of the bowel and surrounding organs, enabling the placement of a drain when a conservative approach is indicated or conversion to laparotomy when necrotic bowel or fecal contamination is detected. This study suggests that laparoscopy is extremely valuable in the initial evaluation of necrotizing enterocolitis and can help avoid potentially unnecessary surgery in an already critically ill infant [29].
Post-Surgical Care Innovations
Extensive resection is required to remove necrotic tissue in cases of prolonged ischemic necrosis of the intestine, resulting in functional failure, ultra-short residual intestine length, and structural abnormalities, leading to the severe complication of Short Bowel Syndrome (SBS) in neonates with NEC. A combination of parenteral nutrition and enteral nutrition (EN) is used to manage this condition, with a continuous evaluation and modification of the treatment strategy to accommodate the child’s development and gastrointestinal needs. The aim is to encourage the recovery of the intestines, enabling them to adjust and absorb nutrients more efficiently and prevent the necessity for intestinal transplantation by improving the function of the remaining intestine [30]. Parenteral nutrition (PN) is essential for caring for patients with NEC, both during diagnosis and after surgery. With careful monitoring, nutrient provision should follow current guidelines to avoid deficiencies or overloads. Periodic evaluation of complications related to parenteral nutrition (PN), such as intestinal failure (IF), PN-induced liver disease (PNALD), metabolic bone disease (MBD), and growth failure, is critical to ensure proper recovery and growth [31,32].
Personalized Medicine in NEC
Tailoring Treatment Approaches
Advanced genomic sequencing technologies have opened the door to individualized strategies in NEC management. Cuna et al. [33] performed whole-genome associated analysis, discovering many single nucleotide polymorphisms (SNPs) linked to NEC vulnerability, possibly providing the foundation for tailored risk evaluations [32]. Combining genomic data with clinical factors and biomarkers could improve the capability to anticipate NEC initiation and development. Ng et al. [34] confirmed that integrating plasma protein biomarkers and clinical factors might reliably forecast NEC growth in preterm neonates [33]. Furthermore, Pammi et al. [35] suggest that evaluating intestinal microbiota might contribute to guiding probiotic therapy to reduce newborn NECs [34].
Risk Stratification Models
Risk-based classification frameworks are intended to identify newborns at high risk for NEC and enable preemptive action and continuous monitoring. Tapas et al. [36] built a risk evaluation framework according to clinical and laboratory criteria that accurately anticipated NEC development and the necessity for surgery [35]. In addition, Battersby et al. [37] developed a NEC prediction score based on gestational age, birth weight, and feeding protocol, which could identify high-risk infants and enable tailored prevention [36]. Another avenue for risk stratification is incorporating machine learning models into electronic health records; Hu et al. [38] used artificial intelligence to develop a predictive model to identify high-risk infants [37]. As a result of personalized medicine, NEC management is experiencing a paradigm shift from a generic approach to a tailored approach based on each patient’s risk profile.
Conclusion
Neonatal care continues to be challenged by necrotizing enterocolitis due to its high mortality and morbidity rates. With advances in biomarkers, imaging, and risk prediction tools, earlier and more accurate diagnosis remains crucial to improving outcomes. There are a variety of preventive and therapeutic innovations, such as probiotics and stem cell therapies, that can be used to significantly reduce the burden of this disease. Personalized treatments based on genetic and clinical data are beginning to guide more effective treatments. The implementation of these new strategies into clinical practice may contribute to improved survival rates and long-term health outcomes for infants with NEC.
References
- Eltayeb AA (2023) Necrotizing Enterocolitis. StatPearls.
- Patel RM, Underwood MA (2021) Probiotics and necrotizing enterocolitis. Semin Pediatr Surg 27(1): 39-46.
- Neu J, Pammi M (2018) Necrotizing enterocolitis: The intestinal microbiome and gut inflammation. Front Pediatr 6: 256.
- Niño DF, Sodhi CP, Hackam DJ (2016) Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 13(10): 590-600.
- Frost BL, Modi BP, Jaksic T, Caplan MS (2017) New medical and surgical insights into neonatal necrotizing enterocolitis: A review. JAMA Pediatr 171(1): 83-88.
- Niño DF, Sodhi CP, Hackam DJ (2020) Necrotizing enterocolitis: Linking prematurity, gut, bacterial dysbiosis, and inflammation in the pathogenesis of NEC. Semin Perinatol 44(7): 151285.
- (2024) Anonymous. About Necrotizing Enterocolitis (NEC). National Institute of Child Health and Human Development.
- (2021)Cleveland Clinic. Necrotizing Enterocolitis (NEC). Cleveland Clinic.
- Tanner SM, Berryhill TF, Ellenburg JL (2015) Pathogenesis of necrotizing enterocolitis. Am J Pathol 185(1): 4-16.
- (2024) Neonatal necrotizing enterocolitis: Clinical features and diagnosis.
- Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F (2023) Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern Emerg Med.
- Selvakumar D, Evans D, Coyte KZ (2022) Understanding the development and function of the gut microbiota in health and inflammation. Frontline Gastroenterol 13(e1).
- Jones MW, Smith A, Davis L (2020) The role of biomarkers in the diagnosis and prediction of necrotizing enterocolitis. Neonatology J 108(3): 251-261.
- Patel RM, Johnson TJ, Nguyen K (2021) Innovations in point-of-care diagnostics for early detection of NEC. Pediatr Res 89(4): 523-530.
- Sharma R, Hudak ML (2022) Artificial intelligence in the prediction of necrotizing enterocolitis: A frontier in neonatal care. J Neonatal Med 117(1): 45-53.
- Zhou L, Tang X, He Y (2022) Role of gut microbiota in neonatal necrotizing enterocolitis. J Pediatr Surg 57(11): 2254-2261.
- Neu J, Walker WA (2011) Necrotizing enterocolitis. N Engl J Med 364(3): 255-264.
- Sodhi CP, Wipf P, Yamaguchi Y (2021) The role of probiotics in the prevention of necrotizing enterocolitis. Front Nutr 8: 667188.
- Elgin TG, Kern SL, McElroy SJ (2021) Development of the neonatal intestinal microbiome and its association with necrotizing enterocolitis. Clin Ther 43(5): 759-772.
- Ahanchi NS, Askenazi DJ (2022) Necrotizing enterocolitis in neonates: Prognosis, diagnosis, and treatment options. Front Pediatr 10: 954735.
- Molloy EJ, Di Fiore JM (2010) Necrotizing enterocolitis: Recent advances and future directions. Trop Pediatr 56(6): 373-381.
- Hunter CJ, Upperman JS, Ford HR, Camerini V (2008) Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Clin Perinatol 35(2): 275-292.
- Drucker NA, McCulloh CJ, Li B (2018) Stem cell therapy in necrotizing enterocolitis: current state and future directions. Stem Cell Res Ther 9(1): 239.
- Ledbetter DJ, Juul SE (2000) Erythropoietin and the incidence of necrotizing enterocolitis in infants with very low birth weight. J Pediatr Surg 35(2): 178-182.
- Maynard AA, Dvorak K, Khailova L (2010) Epidermal growth factor reduces autophagy in intestinal epithelium and the rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299(3): G614-G622.
- Zhou Y, Wang H, Liang L, Zhao WC, Chen Y (2015) Clinical significance of peroxisome proliferator-activated receptor γ and TRAP220 in patients with operable colorectal cancer. World J Gastroenterol 21(6): 1805-1813.
- Smith J, Thyoka M (2013) What role does laparoscopy play in the diagnosis and immediate treatment of infants with necrotizing enterocolitis? J Laparoendosc Adv Surg Tech A 23(4): 397-401.
- Montalva L, Incerti F, Qoshe L, Haffreingue A, Marsac L, et al. (2023) Early laparoscopic-assisted surgery is associated with decreased post-operative inflammation and intestinal strictures in infants with necrotizing enterocolitis. J Pediatr Surg 58(4): 708-714.
- Leva E, Di Cesare A, Canazza L, Arnoldi R, Macchini F, et al. (2010) The role of laparoscopy in newborns affected by NEC. J Laparoendosc Adv Surg Tech A 20(2): 187-189.
- Clark C, Mackinlay GA (2006) Laparoscopy as an adjunct to peritoneal drainage in perforated necrotizing enterocolitis. J Laparoendosc Adv Surg Tech A 16(4): 411-413.
- Capriati, Teresa A, Diamanti De Ville de Goyet (2019) New Nutritional And Therapeutic Strategies Of Nec. Curr Pediatr Rev 15(2):92-105.
- Guiducci S, Duci M, Moschino L, Meneghelli M, Fascetti Leon F, et al. (2022) Providing the Best Parenteral Nutrition before and after Surgery for NEC: Macro and Micronutrients Intakes. Nutrients 14(5): 919.
- Cuna A, George L, Sampath V (2018) Genetic predisposition to necrotizing enterocolitis in premature infants: current knowledge, challenges, and future directions. Semin Fetal Neonatal Med 23(6): 387-393.
- Ng PC, Ma TPY, Lam HS (2015) The use of laboratory biomarkers for surveillance, diagnosis and prediction of clinical outcomes in neonatal sepsis and necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed 100(5): F448-F452.
- Pammi M, Cope J, Tarr PI (2017) Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5(1): 31.
- Tepas JJ, Sharma R, Leaphart CL, Celso BG, Pieper P (2010) Timing of surgical intervention in necrotizing enterocolitis can be determined by trajectory of metabolic derangement. J Pediatr Surg 45(2): 310-314.
- Battersby C, Longford N, Costeloe K, Modi N (2017) Development of a gestational age-specific case definition for neonatal necrotizing enterocolitis. JAMA Pediatr 171(3): 256-263.
- Hu Y, Wang JH, Wang S (2022) Risk factors for necrotizing enterocolitis in preterm infants: a systematic review and meta-analysis. Front Pediatr 10: 818880.






























