- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Biological Therapies in the Management of Ulcerative Colitis: A Comprehensive Analysis and Future Perspectives
Miguel Eduardo Rodriguez Rodriguez1, Ronald Mauricio Blanco Montecino2, Jesus Alejandro Cordova Guilarte1, Kevin J Lopez Romero2, Parajuli Santosh3, Shahtaj A Shah4, Niragh Sikdar5, Ameer Mustafa Farrukh6, Valeria Di Stefano Perez7, Juan Carlos Pantoja8 and Maria Isabel Gomez Coral9*
1Universidad de Oriente, Venezuela. Larkin Community Hospital, Florida
2Universidad de El Salvador, El Salvador
3Kathmandu University, Nepal Medical College, Nepal
4Jinnah Medical and Dental College, Pakistan
5Medical College & Hospital Kolkata, India
6University of Galway School of Medicine, Ireland
7Universidad de Ciencias Médicas de Centro América, Costa Rica
8Universidad del Norte, Barranquilla, Colombia
Submission:December 07, 2023; Published:December 14, 2023
*Corresponding author: Maria Isabel Gomez, Universidad Del Valle De México, México, Email: mariaisagcoral@gmail.com
How to cite this article: Miguel Eduardo Rodriguez R, Ronald Mauricio Blanco M, Jesus Alejandro Cordova G, Kevin J Lopez R, Parajuli S, et al. Biological Therapies in the Management of Ulcerative Colitis: A Comprehensive Analysis and Future Perspectives. Adv Res Gastroentero Hepatol, 2023; 20(2): 556033. DOI: 10.19080/ARGH.2023.20.556033.
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Abstract
This article comprehensively examines ulcerative colitis (UC), a prevalent form of inflammatory bowel disease. The exploration begins with a detailed overview of UC's epidemiology, emphasizing its global prevalence, age distribution, and the rising incidence over recent decades. The pathophysiological section sheds light on the intricate interplay of genetic, environmental, and immune factors contributing to UC, focusing on the disease's chronic inflammatory nature and variable clinical course. Histopathological, the Montreal Classification is dissected to delineate UC's unique features, including its distinct distribution within the colon and characteristic mucosal alterations. The clinical presentation and diagnosis section elucidates hallmark symptoms, extraintestinal manifestations, and the crucial role of endoscopic and histologic findings in confirming UC diagnosis. The subsequent segments delve into the evolving landscape of biological therapies, focusing on tumor necrosis factor (TNF) inhibitors (Infliximab, Adalimumab, Golimumab) and the integrin inhibitor Vedolizumab. Mechanisms of action, dosing regimens, and potential adverse effects are thoroughly explored, providing a comprehensive guide for clinicians navigating therapeutic decisions. Including the IL-12/IL-23 inhibitor, Ustekinumab adds a novel dimension, highlighting its efficacy, safety, and potential cost-effectiveness. This comprehensive resource offers a holistic understanding of UC, contributing to the ongoing pursuit of enhanced patient outcomes in managing this complex inflammatory bowel disease.
Keywords: Ulcerative colitis, Inflammatory bowel disease, Biological therapies; Diagnosis hinges
Abbreviations: UC: Ulcerative Colitis; IBD: Inflammatory Bowel Disease; TNF: Tumor Necrosis Factor; FDA: Food and Drug Administration; IgG1: Immunoglobulin G1; IL-12: Interleukin-12; IL-23: Interleukin-23; ESR: Erythrocyte Sedimentation Rate; CRP: C-Reactive Protein; GALT: Gut-Associated Lymphoid Tissue; IFX: Infliximab; ADA: Adalimumab; GLM: Golimumab; VDZ: Vedolizumab; UST: Ustekinumab
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Introduction
>Ulcerative colitis (UC), a prominent form of inflammatory bowel disease (IBD), is distinguished by persistent and diffuse inflammation limited to the colonic mucosa, extending proximally from the rectum. Globally recognized as the most prevalent variant of IBD, its incidence ranges from 9 to 20 cases per 100,000 persons annually, with a prevalence of 156 to 291 cases per 100,000 persons each year. While the condition can manifest at any age, it is most frequently diagnosed during the second and third decades of life. Various risk factors contribute to its onset, primarily associated with disruptions in intestinal mucosa barriers and subsequent perturbations in the intestinal microbiota, encompassing factors such as infections, genetic predisposition, medication use, tobacco consumption, and mucosal immune dysregulation [1-3]. The hallmark symptom of ulcerative colitis is bloody diarrhea, often accompanied by mucus, with additional manifestations including urgency or tenesmus, abdominal pain, malaise, weight loss, and fever. The severity and extent of the disease dictate the presence of associated symptoms. Extraintestinal manifestations linked to disease activity encompass episcleritis, scleritis and uveitis, peripheral arthropathies, erythema nodosum, and pyoderma gangrenosum. Diagnosis hinges on presenting symptoms consistent with the disease and findings from sigmoidoscopy or colonoscopy, revealing continuous colonic inflammation that originates in the rectum. Confirmatory biopsies exhibit chronic inflammatory changes within the colon. Treatment decisions for ulcerative colitis patients hinge on the disease's extent and severity, incorporating a spectrum of therapeutic options, including biological therapies such as TNF inhibitors (infliximab, adalimumab, golimumab) and integrin inhibitors (vedolizumab) [1,2]. This article seeks to provide a comprehensive overview of the biological therapy landscape for ulcerative colitis, shedding light on its efficacy, mechanisms, and implications for clinical practice.
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Epidemiology & Physiopathology
>Ulcerative colitis is a chronic inflammatory bowel disease characterized by inflammation of the colonic mucosa, typically affecting the rectum and extending proximally continuously. The exact etiology of UC remains elusive, but it is believed to result from a complex interplay of genetic, environmental, and immune factors. Multiple genetic susceptibility loci have been identified, implicating a genetic component in UC's pathogenesis [4,5]. Environmental factors such as alterations in the gut microbiota, dietary habits, and exposure to certain infections may trigger or exacerbate the disease [4]. Dysregulation of the immune response plays a pivotal role in UC, with an aberrant inflammatory response directed against the intestinal mucosa [6]. The epidemiology of UC shows a variable global distribution, with a higher incidence reported in Westernized countries such as the United States and Europe. The incidence and prevalence of UC have been rising over the past few decades, suggesting a potential role for environmental factors in disease development. UC typically manifests in young adulthood, with a bimodal age distribution, although it can occur at any age [5-8]. The disease course of UC is characterized by periods of exacerbation and remission. Clinical manifestations include diarrhea, bloody stools, abdominal pain, and weight loss. Extraintestinal manifestations may also affect the joints, skin, and eyes. Complications such as colonic perforation and toxic megacolon can be life-threatening [4-6]. Endoscopic evaluation and histopathological examination of colonic biopsies are essential for diagnosing UC, with characteristic features including diffuse inflammation, crypt abscesses, and architectural distortion of the colonic mucosa. Several classification systems have been developed to determine the extent and severity of the disease, such as the Montreal classification [4-8].
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Histopathology
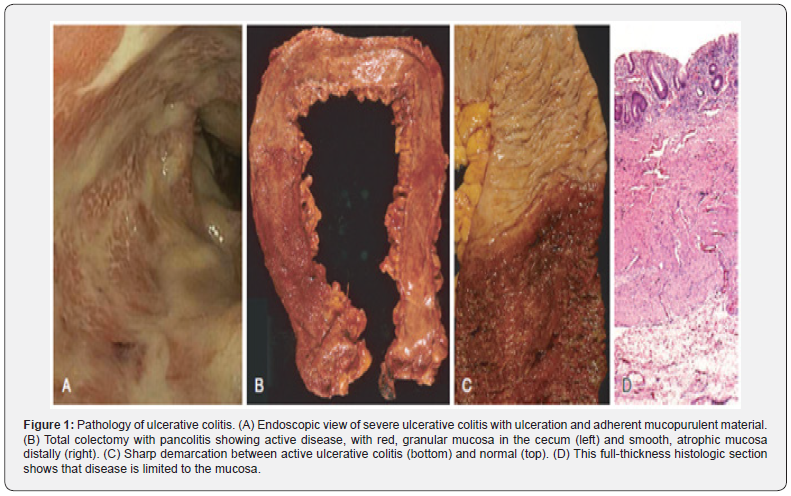
>Ulcerative Colitis is distinguished from other Inflammatory Bowel Diseases given its anatomic distribution. Macroscopically, it is a condition located within the colon and rectum in a diffuse, continuous manner [9-11]. The Montreal Classification classifies the extent of the disease and is done according to the distribution of the involvement [11]. When the entire colon is affected, it is referred to as pancolitis. In contrast, when limited to the rectum or rectosigmoid, it can be called ulcerative proctitis or ulcerative proctosigmoiditis, respectively [9]. Skip lesions, though rare, may occur, and it’s essential to consider them to distinguish them from other inflammatory bowel diseases. The diseased vs. unaffected mucosa is very likely identified on gross anatomy examination. The mucosa has a granular appearance with a red characteristic aspect and may exhibit extensive broad-based ulcers in a longitudinal relation to the Teniae coli [11]. Small pseudo polyps can form due to areas of regenerating mucosa that project into the lumen [9]. In advanced chronic disease, the mucosa may atrophy, lose its haustra, and dilate due to continuing damage by inflammatory mediators to the muscularis propria that disturb the neuromuscular function, leading to toxic megacolon with a significant risk of perforation [9].
>Histologically, it is important to identify the enteric mucosal architecture, lamina propria and submucosa presentation, neutrophilic changes, and epithelial abnormalities for correct and proper diagnosis [11]. The mucosal architecture is distorted by crypt abscess formation and distortion where irregular, dilated, branched, and short crypts form. The disease is extended only to mucosa and submucosa, contains superficial broad-based ulcerations, moderate to elevated lymphoid reaction associated with normal gut-associated lymphoid tissue or GALT, and mild to no fibrosis [9,11]. Inflammatory infiltrates are predominantly by lymphocytes, plasma cells, and granulocytes and are the causal agents for distorting the lamina propria. Neutrophilic granulocyte elevation is mainly seen in exacerbations or flares of Ulcerative colitis with mucosal edema and friable tissue. They are found in the lamina propria, crypt surfaces, and lumen of crypt abscesses. They should not be identified in the normal mucosa [10,11]. There is no granuloma formation, fistulas, or serositis, with the latter only present in severe features of UC. In severe cases, ulcers can extend to the submucosa. Other epithelial abnormalities to be aware of are mucin depletion, such as fewer goblet cells or intracellular mucin; metaplastic changes, such as Paneth cell metaplasia or pyloric gland metaplasia; and epithelial damage, such as flattening, erosions, and ulcerations that manifest in regard to the current stage of presentation of the disease [11]. Figure 1 adapted from: Kumar V, Abbas AK, Aster JC, & Perkins JA Robbins basic pathology. Elsevier.
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Clinical Presentation and Diagnosis
>Ulcerative colitis commonly present as bloody diarrhea that may or may not contain mucus, abdominal pain typically relieved upon defecation, and the sensation of rectal urgency and tenesmus. A small group of patients may also present with constipation [12]. The onset of symptoms varies, ranging from a sudden to a gradual onset. Roughly one-third of the patients with ulcerative colitis suffer from extraintestinal manifestations, which may be present when the disease is inactive. The most common extraintestinal manifestations seen with ulcerative colitis include arthritis (21%), aphthous stomatitis (4%), primary sclerosis cholangitis (4%), uveitis (4%), erythema nodosum (3%), ankylosing spondylitis (2%), pyoderma gangrenosum (2%), and psoriasis (1%) [13].
>The diagnosis of ulcerative colitis is based on a combination of symptoms, endoscopic evidence of continuous colonic inflammation typically starting in the rectum, and histologic findings limited to the mucosal layers. Other histologic findings include varying degrees of infiltrates with immune cells such as lymphocytes, plasma cells, and granulocytes and deformation in the crypt architecture, including crypt atrophy and disarray, crypt abscesses, and branching of the crypts. A common indicator of a chronic inflammatory process is the presence of Paneth cell metaplasia. Importantly, the diagnosis is also favored after ruling out alternative colitis diagnoses, such as infection. Patients suspected of having ulcerative colitis should have stool assessments such as stool culture and clostridium difficile assay. A biopsy is typically confirmatory and can reveal chronic active colitis. Whether a serological marker or a panel of markers alone can diagnose ulcerative colitis is yet to be determined. However, laboratory testing can be helpful in the process, as lab abnormalities commonly correlate with increasing severity or extent of the disease. Inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) may be elevated, but normal levels do not rule out the diagnosis. Fecal calprotectin or fecal lactoferrin may be more sensitive and specific to intestinal inflammation. However, it is essential to note that these tests are not specific for ulcerative colitis and can be abnormal in any case, causing intestinal inflammation or infection [14,15].
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Inhibitors of TNF (Infliximab)
>Infliximab, a chimeric monoclonal antibody consisting of 75% human and 25% murine sequences, exhibits heightened specificity and affinity for TNF-alpha. Its mechanism neutralizes TNF-alpha's biological activity by impeding its binding to receptors. The intravenous administration of an induction dose of 5mg/kg at zero, two, and six weeks is prescribed for patients with moderately to severely active ulcerative colitis (UC) [16-18]. Subsequent maintenance therapy entails 5 mg/kg of repeat infusions, typically every eight weeks, for those demonstrating a satisfactory response based on clinical, endoscopic, and laboratory criteria to sustain remission. In instances of disease flares during maintenance dosing, dosage adjustments can be implemented by either reducing the dosing interval (e.g., from eight weeks to six weeks) or escalating the dose (e.g., from 5 mg/kg to 10 mg/kg). The upper limit for infliximab dosage is 10mg/kg every four weeks. The utilization of TNF-alpha inhibitors, such as infliximab, carries a spectrum of potential adverse events, encompassing infection, malignancy, induction of autoimmunity, demyelinating disease, exacerbation, or new onset heart failure, injection site reactions, neutropenia, and infusion reactions [19]. Contraindications encompass active, untreated infection, latent tuberculosis, demyelinating diseases (e.g., multiple sclerosis, optic neuritis), uncontrolled heart failure, and malignancy. Regarding usage during pregnancy, the experience with anti-TNF agents for IBD treatment is reassuring [20]. The Toronto Consensus on Management of IBD in Pregnancy recommends the continuation of anti-TNF monotherapy throughout pregnancy for pregnant patients [21].
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Inhibitors of TNF (Adalimumab)
>Adalimumab, a pivotal therapeutic agent in managing Ulcerative Colitis (UC), operates through its targeted inhibition of tumor necrosis factor-alpha (TNF-α), a key mediator of inflammatory responses. The recommended dosing regimen involves an initial induction phase with a loading dose of 160mg at Week 0, followed by 80mg at Week 2. Subsequently, a maintenance phase entails administering 40mg every other week. However, dosages may be adjusted based on individual patient responses and clinical needs. This treatment has demonstrated notable efficacy in inducing and sustaining clinical remission, promoting mucosal healing, and enhancing the overall quality of life for UC patients [1]. Indications for adalimumab use encompass cases of moderate to severe UC in adults who have shown inadequate responses to conventional therapies or have experienced intolerance to such treatments. Its role is particularly crucial when conventional options like corticosteroids or immunomodulators have proven insufficient. Despite its effectiveness, carefully considering potential adverse effects is paramount [22,23]. Those adverse effects include injection-site reactions, upper respiratory tract infections, and headaches. Notably, there is an elevated risk of serious infections such as tuberculosis and invasive fungal infections. Also, Adalimumab is contraindicated in individuals with known hypersensitivity to the drug and those with active infections. A comprehensive risk-benefit analysis, thorough patient evaluation, and vigilant monitoring are essential to clinical decision-making when considering Adalimumab therapy for UC [23,24]. Additionally, studies have suggested that Adalimumab, like other TNF-α inhibitors, may cross the placenta and be detected in the neonatal circulation. However, the clinical significance of this transplacental passage and its potential impact on fetal development remain areas of ongoing research. The PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry, a large prospective cohort study, assessed the safety of Adalimumab and other anti-TNF agents during pregnancy. The findings suggested that exposure to Adalimumab during pregnancy was not associated with an increased risk of adverse maternal or fetal outcomes, such as preterm birth, low birth weight, or congenital abnormalities. It is important to note that while these findings are reassuring, the decision to use Adalimumab during pregnancy should be carefully individualized [25].
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Inhibitors of TNF (Golimumab)
>In 2013, Golimumab received approval from the Food and Drug Administration and European Medicines Agency for the treatment of moderately to severely active ulcerative colitis in adults who did not respond well to or could not tolerate conventional therapies such as amino salicylates, oral corticosteroids, azathioprine, or 6-mercaptopurine, or who had become dependent on corticosteroids. Golimumab is a human monoclonal antibody of the IgG1 class. It is administered subcutaneously and has a high affinity for binding to and neutralizing human TNF-alpha. What allows less frequent dosing than other available anti-TNF agents [26]. The induction doses of Golimumab are given two weeks apart and consist of 200mg followed by 100mg. Maintenance doses of 50 or 100 mg are given at 4-week intervals [27].
>The PURSUIT-SC clinical trials aimed to determine the effectiveness of subcutaneous Golimumab in treating ulcerative colitis. The PURSUIT-SC induction study found a positive correlation between serum golimumab concentration and clinical response, indicated by improvements in Mayo score, clinical response, and clinical remission. The best outcomes were observed in dose regimens of 200 mg/100 mg or 400/200 mg at weeks 0 and 2, with 6-week clinical remission rates of 17.8% and 17.9%, respectively, compared to placebo (6.4%) [3]. Subsequently, the PURSUIT-SC maintenance study explored the safety and efficacy of golimumab maintenance therapy in patients who had responded to the induction therapy. The study revealed that patients on a maintenance dose of 50 and 100 mg could maintain higher clinical response through week 54 compared to placebo. Moreover, the patients in the 100 mg group showed significantly higher rates of clinical remission and mucosal healing at weeks 30 and 54 compared to placebo [28,29]. During the PURSUIT maintenance study, the most reported adverse events were ulcerative colitis flares, which led to the discontinuation of the study agent in 1.9% of patients in the placebo group, 3.9% in the Golimumab 50 mg group, and 4.5% in the Golimumab 100 mg group. Other adverse events reported included nasopharyngitis, headache, arthralgia, abdominal pain, upper respiratory tract infection, rash, pharyngitis, and cough. In total, 63.9%, 72.7%, and 73.9% of patients who received placebo, Golimumab 50 mg, or Golimumab 100 mg experienced one or more adverse events. Noteworthy is that adverse events were more common in the golimumab 100 mg group [29].
>Greywoode et al. [30] conducted a post hoc analysis of phase 2 and 3 randomized clinical trials in ulcerative colitis and examined the racial differences in response to Golimumab. The study found that participants from racial minority groups had significantly lower clinical response, clinical remission, and endoscopic healing at week six compared to white participants (adjusted odds ratio [AOR], 0.43; 95% confidence interval [CI], 0.28-0.66 for clinical response, AOR, 0.41; 95% CI, 0.22-0.77 for clinical remission, and AOR, 0.48; 95% CI, 0.31-0.74 for endoscopic healing). Even at week 30, participants from racial minority groups had significantly lower clinical remission and endoscopic healing (AOR, 0.46; 95% CI, 0.28-0.74 for clinical remission and aOR, 0.63; 95% CI, 0.41-0.96 for endoscopic healing). Remarkably, there were no noticeable racial differences in placebo response rates, suggesting a racial difference in Golimumab response. Further research is required to comprehend the repercussions of race on the effectiveness of IBD therapy.
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Integrin Inhibitors (Vedolizumab)
>The biological anti-TNF agents have greatly expanded the treatment options for IBD. However, some patients do not respond or cannot tolerate the effects of this medication. Vedolizumab is a newer medication. It is a fully humanized monoclonal antibody that acts on ɑ4β7 integrin [31]. Note that this medication specifically targets integrins in the gastrointestinal tract. These molecules are involved in cellular movements, such as migrating leukocytes in the gut [32]. Vedolizumab is used in moderately to severely active UC and Crohn’s Disease. Patients are started on an induction dose intravenously. A sample regimen is 300 mg at 0, 2, and 6 weeks. After this period, the dose is given every 8 weeks. Once a satisfactory response is achieved, patients are placed on 300 mg infusion every 8 weeks as maintenance. Monitoring the response to this medication may involve checking C-reactive protein levels or fecal calprotectin levels [32-33]. Vedolizumab is associated with a low incidence of infusion reactions or infections. Patients may also experience symptoms of nasopharyngitis. A severe infection's risk is less than that of other biological agents [32]. Absolute Contraindications for using this medication may include hypersensitivity and active severe infections. Relative contraindications can include a history of recent abdominal surgery [33]. Also, there is no evidence of safety concerns about using vedolizumab and its impact on pregnancy outcomes [34]. Vedolizumab was effective in achieving a clinical response at week 6 at 51% (<35 years), 47% (35-55 years), and 38% (>55 years). By 52 weeks, clinical remission and mucosal healing rates were similar in all age groups [35].
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
IL-12/IL-23 Inhibitor (Ustekinumab)
>Ustekinumab is a human immunoglobulin G1 (IGG1) kappa monoclonal antibody that blocks IL-12 and IL-23. These interleukins play a significant role in inflammation induction; blocking them helps reduce or prevent inflammation progression. The FDA has approved Ustekinumab for the treatment of moderate to severe inflammatory bowel disease in adults who were intolerant to or failed treatment with immunomodulators or corticosteroids but never failed treatment with tumor necrosis factor (TNF) antagonists or who were intolerant to or failed treatment with one or more TNF blockers [36-38]. Ustekinumab has proven efficacy and safety in the induction and maintenance of the treatment of ulcerative colitis [36]. Amiot et al. conducted a multi-center cohort study that revealed Ustekinumab’s efficacy and safety in UC patients. The study included patients’ refractory to other therapies with recurrent drug failures and found Ustekinumab at 12-16 weeks, revealing a steroid-free remission in about one-third of the patients [37]. Ollech et al. emphasized the significant number of patients who have Crohn's disease that were not responding to the standard dose of Ustekinumab of 90 mg subcutaneously every eight weeks. Therefore, their retrospective study demonstrated promising improvement by changing the dosing interval to every four weeks instead of receiving the drug every eight weeks [38]. Another retrospective study conducted by Haider et al. demonstrated Ustekinumab effectiveness with dosage escalation every four weeks in patient’s refractory to the eight-week treatment with Ustekinumab. The study concluded that this dosage escalation improves the clinical outcome while reducing the progression of the disease. Furthermore, the study stated that dose escalation improves CRP and albumin levels in Crohn's disease patients. Haider et al. also emphasized the importance of considering the four-week dose escalation before switching biological agents to treat IBD [39].
>The safety profile in pregnancy is yet to be determined [40]. However, Dayan et al. suggested that Ustekinumab is efficacious and safe in pediatric populations suffering from IBD but emphasized the need for more controlled clinical trials to confirm their observations [41]. Hansson-Hedblom et al. stated that Ustekinumab is more cost-effective in the TNF-failure population. The study found that Ustekinumab had a 94% chance of being more cost-effective than adalimumab and a 72% chance of being more cost-effective than vedolizumab. Ustekinumab has displayed an excellent clinical response and long-term efficacy, with some patients achieving remission and improved biomarkers with few side effects [36].
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
Conclusion
>This comprehensive review highlights the intricate landscape of ulcerative colitis (UC), emphasizing its epidemiology, physiopathology, histopathology, clinical presentation, and diagnosis. The article delves into the distinctive features of UC, including its chronic inflammatory nature, genetic and environmental influences, and the variable global distribution of its incidence. The bimodal age distribution and the complex interplay of exacerbation and remission periods underscore the disease's dynamic nature. Histopathological, UC's unique characteristics, such as the diffuse and continuous distribution within the colon, mucosal architecture distortion, and inflammatory infiltrates, are meticulously explored. The Montreal Classification provides a framework for understanding the extent of the disease, further aiding in its diagnosis and classification. The clinical presentation section elucidates the hallmark symptoms and extraintestinal manifestations associated with UC, contributing to a nuanced understanding of the disease's impact on patients. Diagnosis, relying on a combination of symptoms, endoscopic evidence, and histologic findings, is described, emphasizing the importance of ruling out alternative colitis diagnoses. The subsequent sections delve into the landscape of biological therapies, focusing on tumor necrosis factor (TNF) inhibitors such as Infliximab, Adalimumab, and Golimumab. The mechanisms of action, dosing regimens, and potential adverse effects are thoroughly discussed, providing clinicians with a comprehensive overview for informed decision-making.
>Additionally, this article explores the novel integrin inhibitor Vedolizumab, highlighting its specific targeting of gastrointestinal tract integrins and its potential as an alternative for patients unresponsive or intolerant to TNF inhibitors. Including the IL-12/IL-23 inhibitor, Ustekinumab adds depth to the therapeutic landscape, with insights into its efficacy and safety profile in the induction and maintenance of UC treatment. The evolving understanding of its optimal dosing intervals and potential cost-effectiveness compared to other biological agents enriches the discussion. Overall, this article is a valuable resource for clinicians and researchers seeking a comprehensive understanding of UC, its histopathological intricacies, clinical nuances, and the evolving landscape of biological therapies. By elucidating the complex interplay of factors influencing UC and providing a detailed exploration of therapeutic options, the article contributes to the ongoing efforts to enhance patient outcomes and quality of life in managing this challenging inflammatory bowel disease.
- Review Article
- Abstract
- Introduction
- Epidemiology & Physiopathology
- Histopathology
- Clinical Presentation and Diagnosis
- Inhibitors of TNF (Infliximab)
- Inhibitors of TNF (Adalimumab)
- Inhibitors of TNF (Golimumab)
- Integrin Inhibitors (Vedolizumab)
- IL-12/IL-23 Inhibitor (Ustekinumab)
- Conclusion
- References
References
- Lynch WD, Hsu R (2023) Ulcerative Colitis. In: StatPearls. StatPearls Publishing, Treasure Island (FL), USA.
- Feuerstein JD, Moss AC, Farraye FA (2019) Ulcerative Colitis. Mayo Clin Proc 94(7): 1357-1373.
- Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, et al. (2020) Ulcerative colitis. Nat Rev Dis Primer 6(1): 1-20.
- Khor B, Gardet A, Xavier RJ (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474(7351): 307-317.
- Molodecky NA, Soon IS, Rabi DM, William AG, Mollie F, et al. (2012) Increasing incidence and prevalence of inflammatory bowel diseases with time, based on systematic review. Gastroenterol 142(1): 46-54.
- Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ (2012) Ulcerative colitis. Lancet 380(9853): 1606-1619.
- Fakhoury M, Negrulj R, Mooranian A, Al-Salami H (2014) Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res 7: 113-120.
- Kaplan GG, Ng SC (2016) Globalisation of inflammatory bowel disease: perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol Hepatol 1(4): 307-316.
- Kumar V, Abbas AK, Aster JC, Perkins JA (2018) Robbins basic pathology. Elsevier.
- Lynch Whitney D, Ronald Hsu (2023) Ulcerative Colitis. StatPearls - NCBI Bookshelf.
- Kellermann, Lauge, Lene Riis (2021) A Close View on Histopathological Changes in Inflammatory Bowel Disease, a Narrative Review. Digestive Med Res 4: 3.
- Gajendran M, Loganathan P, Jimenez G, Catinella AP, Ng N, et al. (2019) A comprehensive review and update on ulcerative colitis. Dis Mon 65(12): 100851.
- Adams SM, Bornemann PH (2013) Ulcerative colitis. Am Fam Physician 87(10): 699-705.
- Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF (2017) Ulcerative colitis. Lancet 389(10080): 1756-1770.
- Feuerstein JD, Cheifetz AS (2014) Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc 89(11): 1553-1563.
- Probert CS, Hearing SD, Schreiber S, Kühbacher T, Ghosh S, et al. (2003) Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut 52(7): 998-1002.
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, et al. (2005) Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353(23): 2462-2476.
- Taxonera C, Barreiro-de Acosta M, Calvo M, Saro C, Bastida G, et al. (2015) Infliximab Dose Escalation as an Effective Strategy for Managing Secondary Loss of Response in Ulcerative Colitis. Dig Dis Sci 60(10): 3075.
- Lichtenstein L, Ron Y, Kivity S, Ben-Horin S, Israeli E, et al. (2015) Infliximab-Related Infusion Reactions: Systematic Review. J Crohns Colitis 9(9): 806-815.
- Chaparro M, Verreth A, Lobaton T, Gravito-Soares E, Julsgaard M, et al. (2018) Long-Term Safety of In Utero Exposure to Anti-TNFα Drugs for the Treatment of Inflammatory Bowel Disease: Results from the Multicenter European TEDDY Study. Am J Gastroenterol113(3): 396-403.
- Nguyen GC, Seow CH, Maxwell C, Huang V, Leung Y, et al. (2016) The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterol 150(3): 734.
- Reinisch W, Sandborn WJ, Hommes DW, Geert DH, Stephen H, et al. (2011) Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomized controlled trial. Gut 60(6): 780-787.
- Sandborn WJ, Rutgeerts P, Enns R, Stephen BH, Jean-Frédéric C, et al. (2007) Adalimumab induction therapy for Crohn's disease previously treated with infliximab: a randomized trial. Ann Intern Med 146(12): 829-838.
- Colombel JF, Sandborn WJ, Rutgeerts P, Robert E, Stephen BH, et al. (2007) Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterol132(1): 52-65.
- Julsgaard M (2019) Pregnancy outcomes in women with inflammatory bowel disease treated with tumor necrosis factor inhibitors and/or thiopurines: follow-up of a nationwide cohort. Aliment Pharmacol Ther 50(9): 1058-1067.
- Shealy DJ, Cai A, Staquet K, Baker A, Lacy ER, et al. (2010) Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor α. Mabs 2(4): 428-439.
- Cunningham G, Samaan MA, Irving PM (2019) Golimumab in the treatment of ulcerative colitis. Therap Adv Gastroenterol 12: 1756284818821266.
- Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, et al. (2014) Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterol 146(1): 85-95.
- Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, et al. (2014) Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterol 146(1): 96-109.
- Greywoode R, Petralia F, Ullman TA, Frederic Colombel J, Ungaro RC (2023) Racial Difference in Efficacy of Golimumab in Ulcerative Colitis. Inflamm Bowel Dis 29(6): 843-849.
- Scribano ML (2018) Vedolizumab for inflammatory bowel disease: From randomized controlled trials to real-life evidence. World J Gastroenterol 24(23): 2457-2467.
- A-Rahim YI, Farrell RJ (2023) Overview of dosing and monitoring of biologic agents and small molecules for treating ulcerative colitis in adults. Shibboleth authentication request.
- Cohn HM, Dave M, Loftus EV (2017) Understanding the Cautions and Contraindications of Immunomodulator and Biologic Therapies for Use in Inflammatory Bowel Disease. Inflamm Bowel Dis 23(8): 1301-1315.
- Yajnik V, Khan N, Dubinsky M, Axler J, James A, et al. (2017) Efficacy and Safety of Vedolizumab in Ulcerative Colitis and Crohn's Disease Patients Stratified by Age. Adv Therap 34(2): 542-559.
- Gara SK, Guntipalli P, Marzban S, Taqi M, Aryal V, et al. (2023) Clinical Outcomes of Ustekinumab in Inflammatory Bowel Disease. Cureus 15(10): e46833.
- Amiot A, Filippi J, Abitbol V, Cadiot G, Laharie D, et al. (2020) Effectiveness and safety of Ustekinumab induction therapy for 103 patients with ulcerative colitis: a GETAID multicentre real-world cohort study. Aliment Pharmacol Ther 51(11): 1039-1046.
- Ollech JE, Normatov I, Peleg N, Wang J, Patel SA, et al. (2021) Effectiveness of Ustekinumab Dose Escalation in Patients with Crohn's Disease. Clin Gastroenterol Hepatol 19(1): 104-110.
- Haider SA, Yadav A, Perry C, Su L, Akanbi O, et al. (2020) Ustekinumab dose escalation improves clinical responses in refractory Crohn's disease. Therap Adv Gastroenterol 13: 1756284820959245.
- Gorodensky JH, Bernatsky S, Afif W, Filion KB, Vinet É (2023) Ustekinumab Safety in Pregnancy: A Comprehensive Review. Arthritis Care Res (Hoboken) 75(4): 930-935.
- Dayan JR, Dolinger M, Benkov K, Dunkin D, Jossen J, et al. (2019) Real World Experience with Ustekinumab in Children and Young Adults at a Tertiary Care Pediatric Inflammatory Bowel Disease Center. J Pediatr Gastroenterol Nutr 69(1): 61-67.
- Hansson-Hedblom A, Almond C, Borgström F, Sly I, Enkusson D, et al. (2018) Cost-effectiveness of ustekinumab in moderate to severe Crohn's disease in Sweden. Cost Eff Resour Alloc 16: 28.






























