A Rare Case of Appendiceal Orifice Mucosal Schwann Cell Hamartoma and Review of Pathology
Shreena Gandhi1, Seth Lipshutz2 and Dhruvan Patel3
1Department of Internal Medicine, University of Kansas, USA
2Department of Internal Medicine, Jefferson Northeast, USA
3Division of Gastroenterology, Mercy Fitzgerald Hospital, USA
Submission:August 01, 2023; Published:August 25, 2023
*Corresponding author: Dhruvan Patel, Division of Gastroenterology, Mercy Fitzgerald Hospital, 1501 Lansdowne Ave, 1st Floor, Darby, PA 19023, USA, Email: pateldhruvan@gmail.com
How to cite this article: Shreena G, Seth L, Dhruvan P. A Rare Case of Appendiceal Orifice Mucosal Schwann Cell Hamartoma and Review of Pathology. Adv Res Gastroentero Hepatol, 2023; 20(1): 556027. DOI: 10.19080/ARGH.2023.20.556027.
Abstract
Mucosal Schwann Cell Hamartoma, or MSCH, was coined in 2009 by Gibson and Hornick. This term described a group of neuronal polyps found in the gastrointestinal tract, which are composed of S100 positive neural proliferations that do not contain ganglion cells. After an extensive review of the literature, there are approximately only 36 known cases of Mucosal Schwann Cell Hamartoma. The case that we present is one of a Mucosal Schwann Cell Hamartoma that involves only the appendiceal orifice, which we believe the first case of its kind. Colonoscopies, and the increase in frequency of this procedure being performed within the gastroenterology community, has increased the rate at which new, uncommon, and unique diagnoses are being made. The objective of this report is to present a unique case of “Mucosal Schwann Cell Hamartoma” and highlight the importance of colonoscopy and biopsy in differentiating new and uncommon differential diagnoses to every case. This report also highlights the importance of ruling out other diagnoses, as Mucosal Schwann Cell Hamartoma is a diagnosis of exclusion.
Keywords: Mucosal schwann cell hamartoma; Appendiceal orifice; Colonoscopy; Colorectal cancer; MSCH; Inherited syndromes
Abbreviations: MSCH: Mucosal Schwann Cell Hamartoma; NFP: Neurofilament Protein; MEN2B: Multiple Endocrine Neoplasia 2B
Introduction
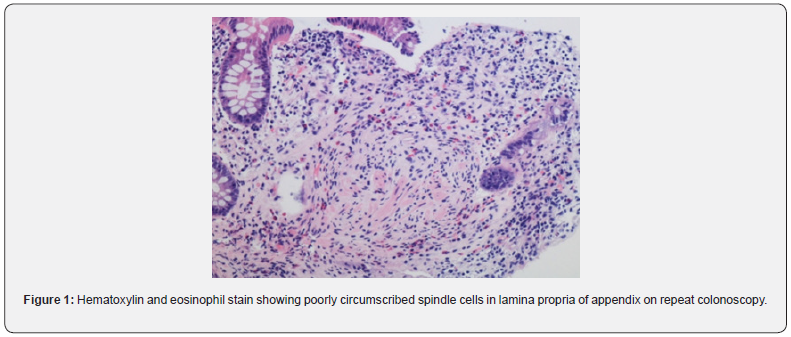
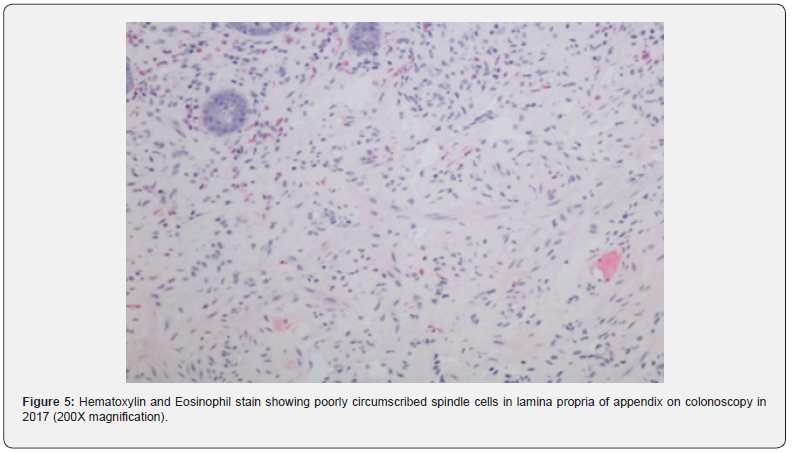
In 2009, Gibson and Hornick coined the new term “Mucosal Schwann Cell Hamartoma” (MSCH) to describe groups of neuronal polyps found in the gastrointestinal tract composed of S100 positive neural proliferations that lack ganglion cells [1]. This entity, distinct from the traditional neuromas and neurofibromas, has been detected more frequently due to the increase in screening colonoscopies in recent years [1]. It is histologically seen as poorly circumscribed, diffuse cellular proliferation of spindle cells in the lamina propria without any whirling, palisading, or fascicular architecture [2]. Based on the review of the total 36 cases known, MSCH has shown no correlation with inherited cancer syndromes [3]. These lesions, though mostly asymptomatic, can also present with occult bleeding or diarrhea [1]. MSCH has been reported most commonly in the sigmoid colon, with a few cases seen in the rectum and the descending colon. The case we present, after extensive literature review, is the first-ever case reported MSCH in the appendiceal orifice (Figure 1).
Results
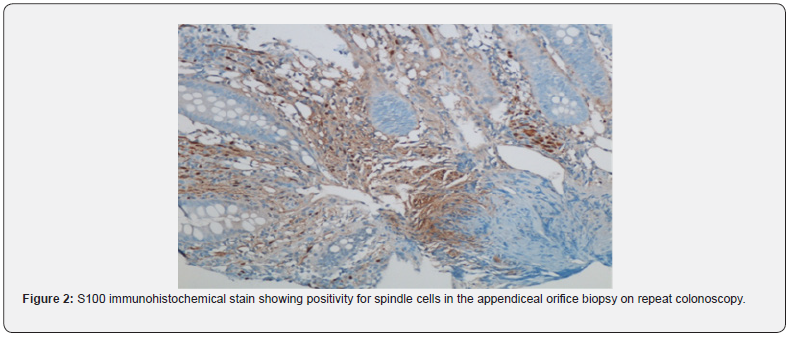
We present a 66-year-old male who underwent an age-determined screening colonoscopy in 2017. Two polyps were detected, one in the cecum (3mm) and another in the transverse colon (6mm), both consistent with Paris classification Is. Biopsy polypectomies confirmed the diagnosis of tubular adenomas. The visualization of the appendix showed a mildly raised area with normal overlying mucosa at the orifice. The biopsy revealed S-100 positive spindle cells in the lamina propria interspersed amongst normal structures. The immunohistochemistry staining was negative for CD34, C-Kit, GFAP, Epithelial Membrane Antigen (EMA), smooth muscle actin, and neurofilament. No discrete mass formation was seen. The findings were consistent with MSCH. Ours is the first-ever case reported of MSCH in the appendiceal orifice. No further investigations were performed. In 2021, the patient underwent repeat screening colonoscopy and re-confirmed the previous findings, and the patient remains asymptomatic (Figure 2).
Discussion
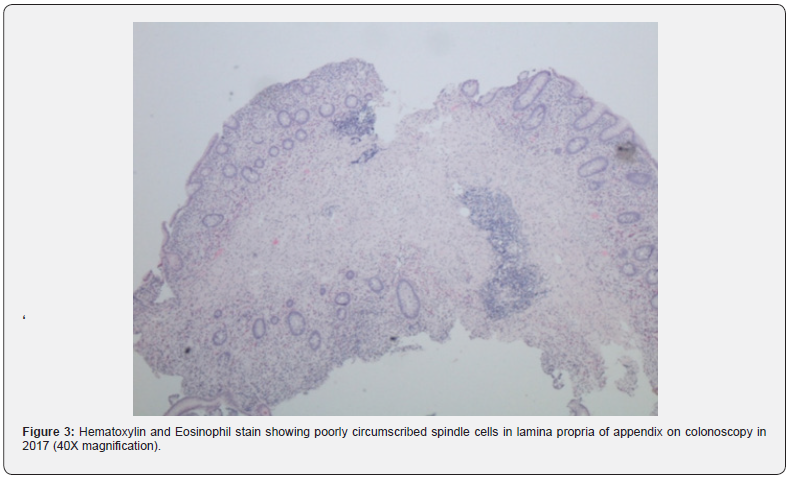
Colonoscopy is the gold standard screening procedure for colorectal carcinoma. Due to an increase in the number of procedures being performed, more atypical pathologies are being encountered [1]. A new term, “Mucosal Schwann Cell Hamartoma” was given by Gibson and Hornick in 2009 after a study of 26 patients of MSCH to avoid confusion with the gastrointestinal lesions seen in the inherited malignancy syndromes [1]. MSCH is a histological diagnosis, and biopsy reveals a benign, poorly circumscribed proliferation of spindle cells with tapering nuclei, dense eosinophilic cytoplasm, indistinct cell borders, and entrapping of the adjacent crypts. No nuclear atypia, pleomorphism, mitotic activity, or ganglion cells have been observed [2]. Cells stain strongly positive for S100 and negative for glial fibrillary acidic protein, epithelial membrane antigen, claudin-1, CD34, smooth muscle actin and KIT markers. Neurofilament protein (NFP) stain showed rare axons in some cases [3] (Figure 3).
The mean age of patients with MSCH is 59.9 years, (54.3 in males, 64.45 in females). The cases reviewed show a female preponderance, with the male to female ratio being 0.8. Most lesions are detected as small polyps, predominantly in the sigmoid colon, followed by rectum, descending colon, transverse, and lastly, ascending colon. The sizes of the polyps range from 1-8mm (mean, 4.5 mm). The majority of the patients were asymptomatic (66.67%), and the lesion was detected on a routine colonoscopy. Some patients presented with gastrointestinal bleeding (14%), abdominal pain and tenesmus (8.3%), and diarrhea (5.5%). None of the cases (0%) presented with the stigmata of inherited cancer syndromes such as neurofibromatosis, MEN2B (Multiple Endocrine Neoplasia 2B), or Cowden syndrome during an average follow up of 48 months. The case we present, after extensive literature review, seems to be the only case reported of MSCH located at the appendiceal orifice (Table 1 & Figure 4).

MSCH is a diagnosis of exclusion and should be made after ruling out similar lesions. The pathology of various gastrointestinal neural lesions is listed below [1,3]. Gastrointestinal stromal tumors (GIST) are the most common spindle cell tumor of the lower gut. Its characteristic immunoreactivity for c-Kit leads to an easy diagnosis [3]. Neurofibromas are a pivotal differential diagnosis for MSCH. These are benign nerve sheath tumors that have a neuronal component of modified Schwann cells and non-neoplastic cells such as fibroblasts, perineural-like cells, and axons [1]. They are strongly associated with Von Recklinghausen syndrome (Neurofibromatosis type 1) [1].
Gastrointestinal neurofibromas are most commonly found in the stomach and small intestine, and seldom in the large intestine [3]. Neurofibromas of the appendix are extremely rare, with only four total cases reported [12]. Mucosal neuromas are ill-defined masses of irregularly arranged hyperplastic nerve fibers [3] and are positive for the epithelial membrane antigen stain. Neuromas are almost always seen as a part of multiple endocrine neoplasia syndrome type IIb (MEN2B). Patients with MEN2B syndrome develop medullary thyroid carcinomas at a young age, aiding in an early diagnosis [3] (Figure 5).
GI ganglioneuromas are seen with Cowden syndrome, Juvenile polyposis syndrome, MEN2B (particularly with diffuse ganglioneuromas), and NF13. Histologically, they are composed of hypercellular stroma, S-100–positive Schwann cells, and interspersed ganglion cells with variable neuron-specific enolase staining. The presence of gangliocytes differentiates it from MSCH [3] (Figure 6).

GI schwannomas are composed of bland spindle cells arranged in vague fascicles [2]. Unlike their counterparts from the central nervous system and peripheral soft tissue, the Verocay Bodies are absent, which can lead to confusion with MSCH [2]. However, the characteristic microscopic findings of Antoni A areas with a high cell density, Antoni B areas with a low cell density, and peripheral lymphoid cuffs and infiltrations set it apart [2]. Peri neuromas show Spindle Cells in a whorled growth pattern that can expand the lamina propria [3]. They can appear morphologically similar to MSCH, but unlike MSCH, the colonic epithelium shows serrated architecture with positive markers such as EMA. Also, they do not react with S100 [3]. Lastly, inflammatory fibroid polyps (Vanek’s Tumor) are composed of stellate or spindle-shaped, bland stromal cells arranged in an onion skin-like pattern around blood vessels and mucosal glands [3]. It varies from MSCH as it doesn’t stain for S100, is composed mainly of eosinophil inflammatory cells, and is highly uncommon in colon [3].
To summarize, there’s a large spectrum of neuronal lesions in the GI tract, which need to be delineated using a thorough microscopic study. Accurate recognition plays a salient role in their clinical, therapeutic, and prognostic implications. MSCH, a relatively new entity, should be considered in the differentials. It is now being detected at unusual sites, such as the appendix, and must be carefully diagnosed based on S100 positive spindle cells.
This is necessary to prevent aggressive or unnecessary treatments that are routinely done for malignancy syndromes.
References
- Gibson JA, Hornick JL (2009) Mucosal Schwann cell “hamartoma”: clinicopathologic study of 26 neural colorectal polyps distinct from neurofibromas and mucosal neuromas. Am J Surg Pathol 33(5): 781-787.
- Pasquini P, Baiocchini A, Falasca L, Dante Annibali, Guido Gimbo, et al. (2009) Mucosal Schwann cell “Hamartoma”: a new entity? World J Gastroenterol 15(18): 2287-2289.
- Han J, Chong Y, Kim TJ, Lee EJ, Kang CS (2017) Mucosal Schwann Cell Hamartoma in Colorectal Mucosa: A Rare Benign Lesion That Resembles Gastrointestinal Neuroma. J Pathol Transl Med 51(2): 187-189.
- Rocco EG, Iannuzzi F, Dell’Era A, Monica Falleni, Laura Moneghini, et al. (2011) Schwann cell hamartoma: case report. BMC Gastroenterol 11: 68.
- Sagami S, Fukumoto A, Amano M, Kentaro Yamao, Yoshimasa Hashimoto, et al. (2012) A case of mucosal Schwann cell hamartoma. Nihon Shokakibyo Gakkai Zasshi 109(10): 1776-1783.
- Bae MN, Lee JE, Bae SM, Eun Young Kim, Eun Ok Kim, et al. (2013) Mucosal Schwann-cell hamartoma diagnosed by using an endoscopic snare polypectomy. Ann Coloproctol 29(3): 130-134.
- Neis B, Hart P, Chandran V, Kane S (2013) Mucosal schwann cell hamartoma of the colon in a patient with ulcerative colitis. Gastroenterol Hepatol (N Y) 9(3): 183-185.
- Ferro de Beça F, Lopes J, Maçoas F, Carneiro F, Lopes JM (2014) Tactoid body features in a Schwann cell hamartoma of colonic mucosa. Int J Surg Pathol 22(5): 438-441.
- Klair JS, Girotra M, Agarwal A, Aduli F (2014) Mucosal Schwann cell hamartoma: just benign or more? Int J Colorectal Dis 29: 1597-1598.
- Kanar O, Nakshabendi R, Berry AC (2015) Colonic mucosal Schwann cell hamartoma on incidental screening colonoscopy. J Gastrointestin Liver Dis 24: 411.
- Bae JM, Lee JY, Cho J, Lim SA, Kang GH (2015) Synchronous mucosal Schwann-cell hamartomas in a young adult suggestive of mucosal Schwann-cell harmatomatosis: a case report. BMC Gastroenterol 15: 128.
- Guo L, He K, Xu X, Li G, Li Z, et al. (2014) Giant appendiceal neurofibroma in von Recklinghausen's disease: A case report and literature review. Oncol Lett 8: 1957-1960.






























