Non-Alcoholic Fatty Liver Disease: Current Concepts of a Silent, but Potentially Reversible Disorder
Blanca Estefanie Avalos Quijano1, Miguel Valdes1, Pushan Aggarwal2, Aishwarya Yannamani2, Ronald Mauricio Blanco Montecino3, Jhon Navarro Gonzalez4, Bijaya Pariyar5, Mayra Rebeca Dominguez de Ramirez3,6, Andrea Gabriela Galecio Chao7, Peggie Crisalida Mendoza Robles8, Javier Isaac Solorzano Leon3, Allan Roberto Bueso Pineda9 and Maria Isabel Gomez10*
1Universidad Evangélica de El Salvador, El Salvador
2Kasturba Medical College Manipal, India
3Universidad de El Salvador, El Salvador
4Universidad del Zulia, Venezuela.
5Kist Medical College and Teaching Hospital, Nepal
6Larkin Community Hospital, USA
7Universidad de Guayaquil, Ecuador
8Universidad San Martin de Porres, Peru
9Universidad Tecnológica Centroamericana, Honduras
10Universidad del Valle de México, México
Submission:May 15, 2023; Published:May 24, 2023
*Corresponding author: Maria Isabel Gomez, Universidad del Valle de México, 154 Samson Rd, Frisco, TX, 76081, USA
How to cite this article: Blanca Estefanie Avalos Q, Miguel V, Pushan A, Aishwarya Y, Ronald Mauricio Blanco M, et al. Non-Alcoholic Fatty Liver Disease: Current Concepts of a Silent, but Potentially Reversible Disorder. Adv Res Gastroentero Hepatol, 2023; 19(4): 556019. DOI: 10.19080/ARGH.2023.19.556019.
Abstract
Non-alcoholic fatty liver disease (NAFLD) is a chronic condition characterized by the accumulation of triglycerides in the liver of individuals who consume little to no alcohol. NAFLD is akin to metabolic syndrome, obesity, type 2 diabetes mellitus, insulin resistance, and dyslipidemia, which contribute as crucial risk factors for the appearance and further progression of the disease. NAFLD is also the most common etiology of chronic liver disease worldwide. The high prevalence of this liver complication is estimated to be driven by obesity and diabetes. A recent meta-analysis found the global prevalence of NAFLD in type 2 diabetes as high as 55.5%. NAFLD is a complex disorder with multifactorial etiology and pathophysiology. The progression of NAFLD can lead to non-alcoholic steatohepatitis (NASH), which can progress to cirrhosis or liver cancer. The exact cause of NAFLD is not fully understood, but it is believed to result from the interaction between genetic, environmental, and metabolic factors. The clinical presentation, including symptoms and signs associated with NAFLD, varies depending on the stage or progression of liver damage. In non-alcoholic fatty liver, the clinical presentation is often asymptomatic in its early stages. They may also present with nonspecific symptoms such as fatigue, malaise, or mild right upper abdominal discomfort. The diagnosis is made with a combination of clinical history, laboratory tests, and imaging studies tailored to the liver. The cornerstone of treatment involves implementing lifestyle changes. These include adopting a healthy diet, engaging in regular physical activity, losing weight, and quitting smoking. A small percentage of patients with NAFLD will develop Non-alcoholic steatohepatitis (NASH), associated with faster liver disease progression. Fibrosis development seems to occur more often in patients with NASH and is an essential histological feature associated with its mortality. Mortality rates vary among different subgroups of patients, with variables such as age, comorbidities, and severity of liver disease playing an important role. This narrative review comprehensively overviews this silent but potentially harmful condition.
Keywords: Non-alcoholic fatty liver disease; Non-alcoholic steatohepatitis; Cirrhosis; Prevention; Diagnosis; Treatment
Abbreviations: NAFLD: Non-Alcoholic Fatty Liver Disease; NAFL: Non-Alcoholic Fatty Liver; NASH; Non-Alcoholic Steatohepatitis; US: United States; DM: Diabetes Mellitus; PLPLA3: Patatin-like Phospholipase Domain-Containing Protein 3; HTN: Hypertension; HCC Hepatocellular Carcinoma
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic condition characterized by the accumulation of fat molecules (triglycerides) in the liver of individuals who consume little to no alcohol [1]. It encompasses a spectrum of conditions ranging from simple steatosis (non-alcoholic fatty liver - NAFL) to nonalcoholic steatohepatitis (NASH), which involves inflammation and tissue damage. While NAFL usually presents as a benign condition that may or not progress to more severe liver disease, NASH is a progressive form with liver inflammation, damage, and fat accumulation [2,3]. In addition, NASH can lead to severe complications such as fibrosis, cirrhosis, and liver cancer. NAFLD is highly prevalent in the United States due to the high obesity rates and other metabolic risk factors such as diabetes and dyslipidemia. It is estimated that NAFLD affects around 25% of the general population in the US, making it the most common chronic liver disease [4].
In the early stages, NAFLD patients may be asymptomatic. However, as the disease progresses, individuals may experience fatigue, abdominal pain, weight loss, hepatomegaly, and elevated liver enzymes [5,6]. The diagnosis of NAFLD includes a combination of medical history, physical examination, lab tests, and imaging studies [6,7]. The prevention and treatment for NAFLD implicate a variety of strategies such as lifestyle modifications (i.e., weight loss, healthy diet, exercise), management of underlying risk factors (i.e., diabetes, hypertension, dyslipidemia), and targeted therapies (i.e., pharmacological interventions) [1,8,9]. The specific treatment approach depends on the severity of the disease and the presence of complications. It should be noted that the progression to endstage liver disease can be halted by combining the mentioned therapeutic strategies [10,11]. This review article aims to provide a better overview of this potentially treatable and silent condition to improve its detection and early management.
Etiology & Pathogenesis
Non-alcoholic fatty liver disease is a complex disorder with multifactorial etiology and pathophysiology. The progression of NAFLD can lead to non-alcoholic steatohepatitis (NASH), which can progress to cirrhosis or liver cancer [12]. The exact cause of NAFLD is not fully understood, but it is believed to result from the interaction between genetic, environmental, and metabolic factors. As previously mentioned, NAFLD risk factors include obesity, insulin resistance, type 2 DM, high blood pressure, dyslipidemia, and metabolic syndrome. In the early stages, NAFLD begins with the accumulation of triglycerides within the hepatocytes and Kupffer cells [12,13]. This excessive fat accumulation can result from increased uptake of fatty acids from the blood, increased synthesis of fatty acids within the liver, or impaired breakdown of fats. The accumulated fat leads to hepatic steatosis, which can progress to inflammation and NASH if left untreated. In NASH, there is established tissue damage and liver cell destruction [14].
The exact triggers for the progression from NAFLD to NASH are not entirely understood. However, oxidative stress, mitochondrial dysfunction, adipokines (hormones released by fat cells), insulin resistance, and pro-inflammatory cytokines play essential roles in the development of NASH. Fat accumulation in hepatocytes triggers inflammation, activating immune cells in the liver [14,15]. This inflammatory response can cause liver cell injury, leading to hepatocyte ballooning and apoptosis. Additionally, fibrosis may develop due to the activation of hepatic stellate cells, which produce excessive extracellular matrix proteins. This fibrosis represents the scarring of liver tissue and serves as the precursor to cirrhosis [16,17].
The progression from NASH to cirrhosis or liver cancer involves a combination of factors. An ongoing inflammation, oxidative stress, and persistent liver cell injury contribute to the development of fibrosis and the subsequent progression to cirrhosis [18]. As fibrosis advances, it disrupts the normal liver architecture, impairs blood flow within the liver, and compromises its function. Over time, extensive scarring can lead to cirrhosis, characterized by replacing normal liver tissue with non-functioning scar tissue. In cirrhosis, there is a higher risk of developing liver cancer (particularly hepatocellular carcinoma -HCC) due to the regenerative nodules that form in the scarred liver tissue [13,19].
It is important to note that the progression from NAFLD to NASH and further to cirrhosis or liver cancer is not linear, and individual cases may vary [12,15]. Therefore, regular monitoring, lifestyle modifications, and comprehensive clinical interventions are essential to manage and potentially prevent the progression of these conditions [13].
Epidemiology
Initially, it was considered a “Western disease”; nevertheless, the prevalence of nonalcoholic fatty liver disease (NAFLD) has increased globally in the last three decades; diagnosed by imaging, it was estimated to be 25.24% [20]. NAFLD is the most common etiology of chronic liver disease worldwide. The high prevalence of this liver complication is estimated to be driven by obesity and diabetes. A meta-analysis study by Yonossi et al. [20-22] presents the global prevalence of NAFLD in Type 2 Diabetes as 55.5%.
In The United States, NAFLD is in the top etiologies as the cause of hepatocellular carcinoma and an indication for liver transplants. Simultaneously in the United States, recent data suggest that NAFLD affects 30% of the general population, 58% of overweight people, and 90% of the morbidly obese [23]. In addition, the highest NAFLD prevalence rate was observed in Latin America (44.4%), followed by North Africa and the Middle East (36.5%), South Asia (33.8%), South-East Asia (33.1%), North America (31.2%), East Asia (29.7%), Asia Pacific (28.0%), and Western Europe (25.1%) (3). Therefore, the prevalence of NAFLD has been estimated to range from 2.8% to 46% worldwide and varies depending on the study population and the diagnostic tool used to determine NAFLD (e.g., liver enzymes, imaging, liver biopsy) [24]. The prevalence of nonalcoholic steatohepatitis (NASH) has been estimated to affect 5-7% of the general population. Also, it is reported that it varies according to ethnicity (3)(4). Hispanics had the highest prevalence of NASH, significantly higher than Caucasians. The reasons for this remain unclear. Both lifestyle and genetic predisposition may play a role. Other studies show that a genetic polymorphism in the PNPLA3 gene has been linked to steatosis and hepatocyte injury and may be more common among Hispanics [24].
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome, nowadays is a leading cause of abnormal liver enzymes and has been associated with an increased risk for the development of diabetes and coronary artery disease [20,24]. Consequently, most deaths in NAFLD are due to cardiovascular disease, while overall cancer mortality is also increased in NAFLD. The excess cardiovascular risk in fatty liver has been reported even in populations with relatively low background adiposity as measured by BMI [25]. Difference in prevalence between genders is not clear; along these lines, data shows that no differences in insulin resistance or obesity were found between male and female patients with NAFLD [24]. Another aspect is that aging is an essential epidemiological factor for NAFLD, NASH, and fibrosis [26].
Risk Factors
NAFLD is akin to metabolic syndrome, obesity, type 2 diabetes mellitus, insulin resistance, and dyslipidemia, which contribute as key risk factors of NAFLD [27,28]. Other risk factors may include genetic predisposition, sex and age, race/ethnicity, diet, physical activity, sleep, small bowel bacterial overgrowth, oxidative stress, and sarcopenia [27]. Overall, NAFLD is associated with an unhealthy lifestyle. Evidence suggests that changing an unhealthy lifestyle may reduce transaminase levels and improve NAFLD [28]. Metabolic syndrome includes abdominal obesity, hypertriglyceridemia, hyperglycemia, HDL cholesterol, and high blood pressure. These metabolic syndrome factors correlate with NAFLD; several metabolic syndrome traits have been linked to a greater chance of more severe liver disease. NAFLD has been acknowledged as a hepatic manifestation of metabolic syndrome. Each condition initiates or exacerbates the other [27].
The obesity epidemic has led to an increase in NAFLD, being an established risk factor. The widespread presence of NAFLD among obese individuals is 50%-90% [27]. As weight gain and BMI rise, the NAFLD risk becomes more prominent. Weight loss is the only proven method for recovery of NAFLD [27]. Obesity, insulin resistance, and type 2 diabetes are significant risk factors for the appearance of NAFLD. Insulin resistance creates lipolysis of adipose tissue, which releases free fatty acids in the liver. Much population-based research has stated that NAFLD is more present in individuals with type 2 diabetes; they are also at higher risk for NASH and complications related to the liver, as well as cirrhosis [27]. Another key risk factor for NAFLD is Dyslipidemia. Lipotoxicity plays a significant role in the advancement from mild NAFLD toward steatohepatitis. Lipotoxicity signifies high levels of toxic lipids and derivatives induced by fat content accumulation in non-adipose tissues. Persons with NAFLD have elevated free fatty acids, triglycerides, and other lipids, including free cholesterol, bile acids, lysophosphatidylcholines, and ceramides [27].
Other non-metabolic risk factors, such as genetic predisposition, may occur within individuals. Genetic variants have a prominent place in the appearance and development of NAFLD, such as Patatin-like phospholipase domain-containing 3 in addition to transmembrane 6 superfamily, member 2 singlenucleotide polymorphisms [27,29]. Furthermore, sex and age can also contribute as risk factors, which are seen more in men than women according to their age. The increased risk for men is during middle age, while in women, it is during post-menopause; it also occurs when other medical problems arise with age, such as type 2 diabetes, hypercholesterolemia, and hypertension. For women, estrogen may play a protective role in NAFLD [27]. Other risks are ethnicity related, seen more in Hispanic populations which can also be associated with a genetic predisposition. The 1148M variant of PLPLA3 is associated with NAFLD, followed by Caucasian and African Americans. This latter population has more prevalence of obesity and metabolic syndrome, despite Hispanics having a higher risk of NAFLD [27].
Other risks include a high-caloric diet, low exercise level, poor sleep hygiene, gut microbiota, oxidative stress, and Sarcopenia [27]. High sugar diets promote de novo lipogenesis and produce an inflammatory response causing hepatocyte injury via the c Jun-N-Terminal pathway. Omega 3 and Mediterranean diets have positively prevented and treated NAFLD [27]. Sedentary life also increases the risk for NAFLD. It can be reversed with at least a low level of exercise, helping to normalize liver enzymes in patients with NASH because exercise may reduce insulin resistance and adiponectin levels. Poor sleep is related to obesity which contributes to NAFLD, cytokines such as interleukin 6 and TNFalpha (both associated with their elevation in sleeping problems) increase adipocyte lipolysis causing a high hepatic flow of free fatty acids, and poor sleep also affects the hypothalamic-pituitaryadrenal axis and high cortisol, cause liver fat deposition [27]. Small intestinal bacterial overgrowth, high in patients with NAFLD, may be related to endotoxins contributing to hepatic fat accumulation. Oxidative stress, the production of reactive oxidative species, and lipid peroxidation may decrease antioxidant enzymes and induce hepatocyte damage, which can be reversed with antioxidants such as vitamin E which have shown positive outcomes in patients with steatohepatitis [27,28]. Lastly, Sarcopenia is a condition where strength, skeletal muscle mass, and function are compromised, diagnosed based on the loss of appendicular muscle mass. An extensive report of Sarcopenia has been seen in patients with NAFLD/NASH. And cirrhosis. Sarcopenia has been observed to worsen liver disease and seems to share common underlying factors with NAFLD, such as insulin resistance and chronic inflammation [27].
Clinical Presentation
The clinical presentation, including symptoms and signs associated with NAFLD, varies depending on the stage or progression of liver damage. In non-alcoholic fatty liver, the clinical presentation is often asymptomatic in its early stages [30]. It is typically detected incidentally during routine blood tests or imaging studies. Patients with NAFL commonly exhibit typical manifestations associated with metabolic syndrome (central obesity, DM, HTN). They may also present with nonspecific symptoms such as fatigue, malaise, or mild right upper abdominal discomfort. Mild hepatomegaly may be palpable during physical examination in very few cases [31].
Patients with NASH may experience symptoms similar to those with NAFLD, including fatigue, abdominal discomfort, and hepatomegaly. Additionally, they may have more pronounced symptoms such as jaundice, pruritus, and anorexia. Some individuals may develop signs of chronic liver disease, such as spider nevi blood vessels, palmar erythema, and splenomegaly [32]. In advanced cases, signs of cirrhosis, such as ascites, jaundice, and liver palm, may be present. If left untreated, NASH may progress to cirrhosis, characterized by portal hypertension, ascites, lower extremity edema, hepatic encephalopathy, muscle wasting, gynecomastia, and abdominal collateral vessels. In more advanced stages, hepatocellular carcinoma (HCC) may develop as a complication of long-standing liver insult. Symptoms of HCC include weight loss, abdominal pain, loss of appetite, nausea, jaundice, fatigue, and weakness [33].
It’s important to note that the clinical presentation of these chronic liver conditions can vary among individuals, and some cases may be asymptomatic or present with atypical features.
Diagnosis
NAFLD can be diagnosed by the presence of hepatic steatosis in imaging or histology, no history of alcohol usage, no other etiology accountable for the symptoms, and negative history of chronic liver disease [34]. Therefore, it is essential to begin the evaluation by ruling out other potential conditions. A complete medical history is gathered to determine previous infections, alcohol status, an autoimmune component, associated symptoms, and family history. In addition to the specific biomarkers used in the scoring system, all patients should be tested for hepatitis virus antibodies, plasma iron, ferritin, total iron binding capacity, and an autoimmune liver panel.
Because of the increased risk of comorbidities associated with NAFLD, diagnostic biomarkers are a valuable tool for early detection and treatment. For those biomarkers that have been employed for diagnosis, there are two categories: laboratory tests and imaging, as well as noninvasive and invasive testing [35]. One of the noninvasive diagnostic tools is the Fatty Liver Index (FLI), which uses an algorithm to estimate hepatic steatosis and is a well-predictive tool for NAFLD [35]. The main components of this algorithm are BMI, waist circumference, serum TG and gamma-glutamyl transferase (GGT) measurements. There are several imaging tests used to detect NAFLD, the presence of hepatic steatosis can be shown during an ultrasound: however, computer tomography (CT) or magnetic resonance imaging (MRI), vibration-controlled transient elastography, and magnetic resonance spectroscopy.
Ultrasound
Abdominal ultrasonography accurately detects hepatic steatosis, which presents as a typical hyperechoic liver [35]. Several studies have been conducted to assess the accuracy of ultrasound in detecting hepatic steatosis. In a meta-analysis comparing the accuracy of ultrasonography versus histology, it was found that ultrasound has 84.8% sensitivity and 93.6% specificity for detecting moderate to severe fatty liver [36]. Furthermore, a double-blind prospective study indicated that when there is 20% fat, the sensitivity increases to 100%, and the specificity climbs to 90% [37]. Moreover, the ultrasound findings can be used with fatty liver index and NAFLD liver fat score [36]. Nevertheless, abdominal ultrasound does not accurately detect hepatic steatosis in severely obese patients [38]. Overall, because of the benefits of this noninvasive approach, such as low cost, easy accessibility, and safety due to the lack of radiation exposure, ultrasound is the best first step to stratify NAFLD (Table 1).
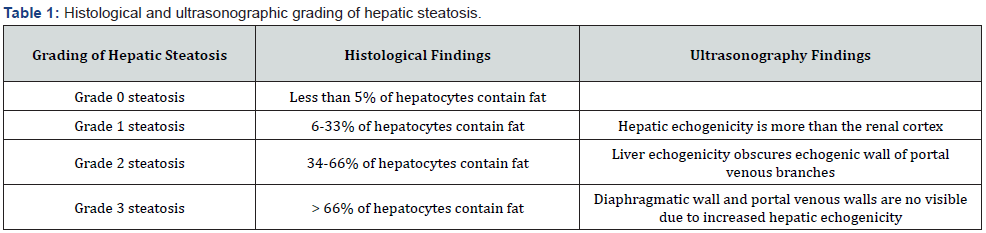
Computer tomography (CT)
CT is an excellent method for detecting hepatic steatosis. Hepatic steatosis is detected when spleen attenuation > liver attenuation. It can also determine moderate to severe hepatic steatosis [39]. However, due to several limitations, such as exposure to radiation and the inability to identify mild steatosis, it is not regarded as the primary method for diagnosing hepatic steatosis [36].
Magnetic resonance and magnetic resonance spectroscopy
MRI is a robust quantitative and qualitative tool for detecting hepatic steatosis. The sensitivity of MRI is 76,7% to 90%, and the specificity is 87%,1 to 91% [39]. Therefore, it is considered as an alternative option for a liver biopsy. Magnetic resonance spectrography accurately identifies changes in hepatic steatosis. Because MRS is not always available in all healthcare systems, MRI is a more dependable option.
Liver biopsy
When the diagnosis is unclear, a liver biopsy is the gold standard for diagnosis since it allows for grading and histological assessment based on fat-containing hepatocytes and the severity of the disease [39]. It should be used in patients with cytopenias, blood ferritin levels that are more than 1.5 times the upper limit of normal, obesity or diabetes Steatosis of more than 5% of hepatocytes is required to diagnose NAFLD [53]. NASH is distinguished from NAFLD by histological findings; thus, NASH is the progression of NAFLD, emphasizing the significance of NAFLD activity; NAS of > or = 5 is associated with a diagnosis of NASH [40] (Table 2).
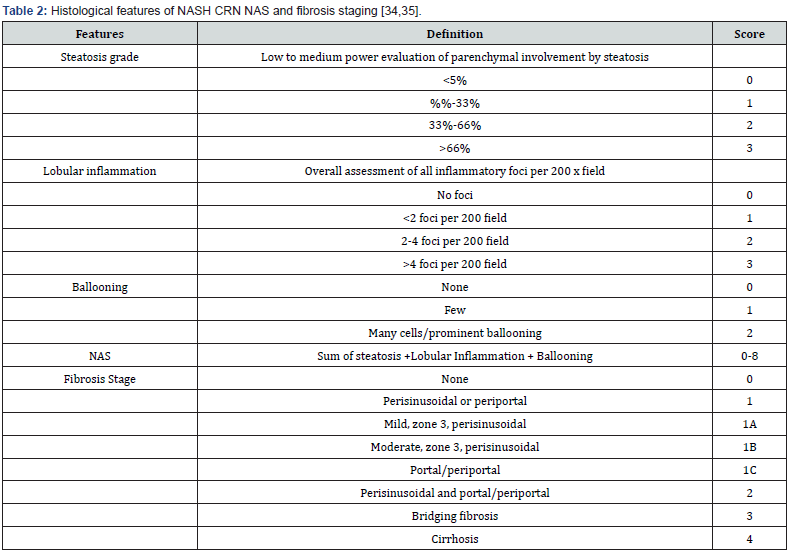
Prevention & Treatment Strategies
NAFLD could result in catastrophic consequences for the patient as it is strongly associated with obesity, insulin resistance, and metabolic syndrome. Fortunately, this chronic liver condition is considered preventable, and several strategies can be employed to reduce the risk of its development [41]. The cornerstone of NAFLD treatment involves implementing lifestyle changes. These include adopting a healthy diet, engaging in regular physical activity, losing weight (if overweight or obese), and avoiding harmful habits such as smoking. First, it is imperative to adopt a well-balanced diet, emphasizing whole grains, fruits, vegetables, and lean proteins while limiting the consumption of saturated fats, trans fats, and refined sugars [42].
Moreover, regular physical activity (aerobic and strength training) and weight management are essential as excess weight, particularly abdominal obesity, is strongly associated with NAFLD [41,43]. In order to avoid or increase liver damage, it is also essential to be cautious with certain medications, such as corticosteroids, tamoxifen, and some anti-inflammatory drugs, as they may contribute to the progression of liver injury. Although NAFLD is unrelated to alcohol consumption, excessive alcohol intake can exacerbate liver damage and worsen the condition. Therefore, it is essential to consume alcohol in moderation or, if possible, avoid it altogether [44].
In addition to preventive strategies, all coexisting contributing conditions must be adequately managed in order to avoid liver damage progression to fibrosis/cirrhosis [44,45]. For example, individuals with diabetes or metabolic syndrome are at a higher risk of developing NAFLD. Proper management of these conditions, including blood glucose control, cholesterol management, and blood pressure regulation, can significantly reduce the risk of fatty liver disease [43,46]. As NAFLD is often associated with metabolic syndrome, diabetes, and cardiovascular disease, it is crucial to address these conditions appropriately. Regular monitoring of liver function through blood tests and imaging studies can help track the progression of NAFLD and assess the effectiveness of treatment strategies [46]. It should be stressed that the prevention and treatment of NAFLD are multifaceted and require a comprehensive approach incorporating primary care providers, hepatologists, nutritionists, and endocrinologists.
Prognosis
A small percentage of patients with NAFLD will develop Nonalcoholic steatohepatitis (NASH), associated with faster liver disease progression. In addition, fibrosis development seems to occur more often in patients with NASH and is an essential histological feature associated with its mortality. Accordingly, NASH has been reported as the leading cause of liver transplants in women and the second in men (following alcohol liver disease) [47]. In a meta-analysis performed on over 8 million patients from 22 countries, the long-term clinical outcomes of NAFLD included advanced fibrosis development, hepatocellular carcinoma, higher liver-specific mortality, and possibly overall mortality, driven mainly by those with underlying NASH [48]. The fibrosis stage represents the most critical variable to consider when assessing long-term prognosis in a patient [49]. Even in the first stages, fibrosis is the most relevant feature associated with liver transplantation, liver-related events, and overall mortality in patients with NAFLD, regardless of other histologic components reported in liver biopsy [49]. In addition, fibrosis stages F3 and F4 have been associated with increased risks of liver-related complications and death [50]. This becomes important in the clinical setting, as the absence of fibrosis is associated with a lower risk of dying or developing liver-related complications within 1 or 2 decades, and its presence helps to identify a subset of patients in which appropriate measures need to be done to achieve a reduction of fibrosis progression [49].
From the total of patients with NAFLD, only a tiny percent will progress to advanced fibrosis and cirrhosis with their associated risk of hepatocellular carcinoma. A systematic review and metaanalysis revealed an increased incidence of hepatocellular carcinoma and extrahepatic cancer in patients with NAFLD, being uterine, breast, prostate, colorectal, and lung cancer as the most related extrahepatic cancers [51]. Unlike hepatocellular carcinoma, in which the degree of fibrosis has a direct relation with its incidence, it does not seem to be the case with extrahepatic cancers [51].
In an effort to understand the natural history of NASH, a Medicare data analysis was performed on over 10 million Medicare Beneficiaries through an 8 year-study period, revealing a substantial cumulative risk (39%) of NAFLD/NASH progression to more severe disease (compensated cirrhosis, decompensated cirrhosis, liver transplant or hepatocellular carcinoma), while patients with compensated cirrhosis at the time of diagnosis had a higher cumulative risk (45%) [52]. In addition, the risk of progression to advanced liver disease has been reported to be significantly higher in patients with comorbidities such as cardiovascular disease, renal impairment, dyslipidemia, and diabetes [52].
Mortality in NAFLD varies among different subgroups of patients, with variables such as age, comorbidities, and severity of liver disease playing an important role. Rates have been reported to vary across the spectrum of liver disease, with NAFLD having the lowest and hepatocellular carcinoma the highest [52]. Cardiovascular disease remains the primary cause of death in patients with NAFLD [53,54].
Conclusion
Non-alcoholic fatty liver disease is a significant and growing health concern worldwide. Its multifactorial etiology involves genetic predisposition, insulin resistance, metabolic syndrome, and lifestyle factors such as diet and physical inactivity. In addition, several risk factors, including obesity, type 2 diabetes, dyslipidemia, and hypertension, contribute to the development and progression of the disease. While NAFLD may initially be asymptomatic, it can progress to more severe stages, including non-alcoholic steatohepatitis and, eventually, cirrhosis or liver cancer. Therefore, early detection and intervention are crucial to prevent further liver damage. Lifestyle modifications, including dietary changes, regular exercise, and weight loss, are pivotal in managing NAFLD. Therefore, these interventions should be encouraged as the primary approach to treatment. However, given the multifaceted nature of NAFLD, a comprehensive approach involving primary care physicians, hepatologists, dietitians, and exercise specialists is crucial to provide holistic care and address various disease aspects. It is crucial to conduct regular monitoring and follow-up appointments to track disease progression, manage comorbidities, and adjust treatment strategies as needed. Early identification of patients at risk of developing cirrhosis allows for timely interventions and reduces the burden of advanced liver disease.
References
- Weiß J, Rau M, Geier A (2014) Non-alcoholic fatty liver disease: epidemiology, clinical course, investigation, and treatment. Dtsch Arztebl Int 111(26): 447-452.
- Powell EE, Wong VW, Rinella M (2021) Non-alcoholic fatty liver disease. Lancet 397(10290): 2212-2224.
- Pallayova M, TaheriS (2014) Non-alcoholic fatty liver disease in obese adults: clinical aspects and current management strategies. Clin Obes 4(5): 243-253.
- Aguilar-Olivos NE, Oria-Hernández J, Ponciano-Rodríguez G, Chávez-Tapia NC, Uribe M, et al. (2015) The Role of Epigenetics in the Progression of Non-Alcoholic Fatty Liver Disease. Mini Rev Med Chem 15(14): 1187-1194.
- Luci C, Bourinet M, Leclère PS, Anty R, Gual P (2020) Chronic Inflammation in Non-Alcoholic Steatohepatitis: Molecular Mechanisms and Therapeutic Front Endocrinol (Lausanne) 11: 597648.
- Maurice J, Manousou P (2018) Non-alcoholic fatty liver disease. Clin Med (Lond) 18(3): 245-250.
- Lonardo A, Sookoian S, Pirola CJ, Targher G (2016) Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism 65(8): 1136-1150.
- Cobbina E, Akhlaghi F (2017) Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev 49(2): 197-211.
- Pappachan JM, Antonio FA, Edavalath M, Mukherjee A (2014) Non-alcoholic fatty liver disease: a diabetologist's perspective. Endocrine 45(3): 344-353.
- Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P (2018) Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci 75(18): 3313-3327.
- Dyson J, Day C (2014) Treatment of non-alcoholic fatty liver disease. Dig Dis 32(5): 597-604.
- Mantzoros CS. (2019) Obesity and Nonalcoholic fatty liver disease: pathophysiology to therapeutics. Metabolism 92: 82-97.
- Kowdley KV (2018) Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin Liver Dis 22(1): 23-37.
- Manne V, Handa P, Kowdley KV (2018) Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin Liver Dis 22(1): 23-37.
- Pinzani M (2019) Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med 65: 37-55.
- Smith A, Baumgartner K, Bositis C (2019) Cirrhosis: Diagnosis and Management. Am a Fam Physician 100(12): 759-770.
- Romanelli RG, Stasi C (2016) Recent Advancements in Diagnosis and Therapy of Liver Cirrhosis. Curr Drug Targets 17(15): 1804-1817.
- Anand BS (1999) Cirrhosis of liver. West J Med 171(2): 110-115.
- Kumar M, Sarin SK (2007) Is cirrhosis of the liver reversible. Indian J Pediatr 74(4): 393-9.
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L et al. (2016) Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1): 73-84.
- Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S (2019) Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation 103(1): 22-27.
- Younossi Z, Golabi P, Paik J, Henry A, Van Dongen C et al. (2023) The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77: 1335-1347.
- Pereira K, Salsa Mendi J, Casillas J (2015) The Global Nonalcoholic Fatty Liver Disease Epidemic: What a Radiologist Needs to Know. J Clin Imaging Sci 5: 32.
- Williams C, Stengel J, Asike M et al. (2011) Prevalence of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among a Largely Middle-Aged Population Utilizing Ultrasound and Liver Biopsy: A Prospective Study. Gastroenterology 140: 124-131.
- Barik A, Shah RV, Spilahari A, et al. (2016) Hepatic steatosis is associated with cardiometabolic risk in a rural Indian population: A prospective cohort study. Int J Cardiol 225: 161-166.
- Mitra S, De A, Chowdhury A (2020) Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol 5: 16.
- Huh Y, Cho YJ, Nam GE (2022) Recent Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease. J Obes Metab 31(1): 17-27.
- Sakran N, Graham Y, Leal A, Pintar T, Yang W (2022) Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord 22(1): 63.
- Kim DY, Park JY (2020) Genetic risk factors associated with NAFLD. Hepatoma Res 6: 85.
- Abraldes JG, Solà E, Fabrellas N, Kamath PS (2021) Liver cirrhosis. Lancet 398(10308): 1359-1376.
- Lai M, Afdhal NH (2019) Liver Fibrosis Determination. Gastroenterol Clin North Am 48(2): 281-289.
- Stasi C (2016) Recent Advancements in Diagnosis and Therapy of Liver Cirrhosis. Curr Drug Targets 17(15): 1804-1817.
- Targher G (2015) NAFLD, a multisystem disease. J Hepatol 62(1 Suppl): S47-64.
- Naga Chalasani, Zobair Younossi, Joel E Lavine, Michael Charlton, Kenneth Cusi, et al. (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1): 328-357.
- Yin X, Guo X, Liu Z, Wang J (2023) Advances in the Diagnosis and Treatment of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci 24(3): 2844.
- Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, et al. (2011) Diagnostic Accuracy and Reliability of Ultrasonography for the Detection of Fatty Liver: A Meta-Analysis. Hepatology 54(3): 1082-1090.
- Dasarathy, S, Dasarathy J, Khiyami A, Joseph R, Lopez R, et al. (2009) Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. Journal of Hepatology 51(6): 1061-1067.
- de Moura Almeida A, Cotrim HP, Barbosa DBV, de Athayde LGM, Santos AS, et al. (2008) Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World Journal of Gastroenterology 14(9): 1415-1418.
- Paul J (2020) Recent advances in non-invasive diagnosis and medical management of non-alcoholic fatty liver disease in adult. Egyptian Liver Journal 10(1): 1-18.
- Tiniakos DG (2009) Liver biopsy in alcoholic and non-alcoholic steatohepatitis patients. Gastroenterol Clin Biol 33(10-11): 930-939.
- Neuschwander-Tetri BA (2017) Non-alcoholic fatty liver disease. BMC Med 15(1): 45.
- Trauner M (2022) Current treatment of non-alcoholic fatty liver disease. J Intern Med 292(2): 190-204.
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO (2016) Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64(6): 1388-1402.
- Day C (2014) Treatment of non-alcoholic fatty liver disease. Dig Dis 32(5): 597-604.
- Maida M, Macaluso FS, Salomone F, Petta S (2016) Non-Invasive Assessment of Liver Injury in Non-Alcoholic Fatty Liver Disease: A Review of Literature. Curr Mol Med 16(8): 721-737.
- Altinbas A, Sowa JP, Hasenberg T, Canbay A (2015) The diagnosis and treatment of non-alcoholic fatty liver disease. Minerva Gastroenterol Dietol 61(3): 159-169.
- Noureddin M, Vipani A, Bresee C, Tsuyoshi Todo, Irene K Kim, et al. (2018) NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 113(11): 1649-1659.
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, et al. (2016) Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1): 73-84.
- Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, et al. (2015) Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 149(2): 389-397.
- Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, et al. (2021) NASH Clinical Research Network (CRN). Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med 385(17): 1559-1569.
- Thomas JA, Kendall BJ, Dalais C, Macdonald GA, Thrift AP (2022) Hepatocellular and extrahepatic cancers in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur J Cancer 173: 250-262.
- Loomba R, Wong R, Fraysse J, Shreay S, Li S, et al. (2020) Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real-world analysis of Medicare data. Aliment Pharmacol Ther 51(11): 1149-1159.
- Bischoff SC, Barazzoni R, Busetto L, Campmans-Kuijpers M, Cardinale V, et al. (2022). European guideline on obesity care in patients with gastrointestinal and liver diseases – Joint European Society for Clinical Nutrition and Metabolism / United European Gastroenterology guideline. United European Gastroenterology Journal 10(7): 663-720.
- Chalasani, N, Younossi Z, Lavine JE, Charlton M, Cusi K, et al. (2018). The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1): 328-357.






























