Prevalence of Infective and Non-Infective Hepatitis in a Tertiary Fever Hospital: Alexandria Governorate, Egypt
Mona A Amin1*, Amr Hosny2 and Maha Abdelhamid2
1Internal Medicine Department, Cairo University, Egypt
2Alexandria Fever Hospital, Egypt
Submission:April 09, 2023; Published:April 21, 2023
*Corresponding author:Mona A Amin, Internal Medicine Department, Cairo University, Egypt, Email: monasleman@hotmail.com
How to cite this article:Mona A A, Amr H, Maha A. Prevalence of Infective and Non-Infective Hepatitis in a Tertiary Fever Hospital: Alexandria Governorate, Egypt. Adv Res Gastroentero Hepatol, 2023; 19(3): 556015. DOI: 10.19080/ARGH.2023.19.556015.
Abstract
Background: Estimates the different etiological varieties of hepatitis, helps to set priorities when deciding policies of investigations and management.
Objective: To estimate prevalence of infective and non-infective causes of hepatitis with or without jaundice in Alexandria Fever Hospital AFH.
Methods: A hospital-based cross-sectional study was conducted at AFH, a tertiary hospital in Alexandria governorate. The Liver Investigations unit (LIU) was responsible for setting the protocol of investigations and management.
Results: The most common causes were autoimmune hepatitis (25.2%) followed by cryptogenic (14.4%). Most patients with toxic hepatitis (Drug induced) (81.8%), autoimmune hepatitis (72.4%), acute HCV (66.7%) were females while most cases with leptospirosis (85.7%), hemochromatosis (83.3%), Bilharziasis (75%) were males.
Conclusion: In acute hepatitis patients, autoimmune hepatitis was the most common cause followed by toxic hepatitis. In chronic hepatitis patients, nonalcoholic steatohepatitis (NASH) was the most common cause followed by metabolic causes.
Keywords: Acute hepatitis; Chronic hepatitis; Non infective hepatitis; Non infective jaundice
Abbreviations: LIU: Liver Investigations Unit; NASH: Nonalcoholic Steatohepatitis; AFH: Alexandria Fever Hospital; SPSS: Statistical Package of Social Science Software; ALF: Acute Liver Failure; NSAIDs: Non Steriodal Anti-Inflammatory Drugs; SAHF: Sub-Acute Hepatic Failure; ACLF: Acute-on-Chronic Liver Failure; WD: Wilson Disease; EASL: European Association for the Study of the Liver
Introduction
Specific causes of parenchymal liver diseases consist of abundant varieties which give rise to illnesses that are similar in their clinical and pathological features. The most common varieties include viral hepatitis, alcoholic, toxic hepatitis, autoimmune hepatitis, non- alcoholic steatohepatitis (NASH), hemochromatosis, Wilson disease and cardiac cirrhosis. Viral hepatitis is almost caused by one of the specific hepatitis viruses (HAV, HBV, HCV, HDV, HEV); hepatitis due to other viruses non-A, including: (EBV, CMV, Yellow fever virus) accounts for only 1-2% of cases [1]. All mentioned hepatitis causes result in acute or chronic hepatitis with abnormal liver function tests and icteric picture in most patients [2]. In a developing country like Egypt, the importance of estimating the prevalence of each illness is pivotal to minimize occurrence of complications, time waste and costs of investigations and treatment. Patients with biochemical liver impairment are commonly admitted to Alexandria Fever Hospital (AFH) with a multitude of causes that might be difficult to distinguish. Every year, about one thousand patients with jaundice and hepatitis are admitted to AFH. About 15-20% (150-200 patients / year) of those patients have negative viral markers for hepatotropic viruses A, B and C, namely HAV-IgM, HBs-Ag and HCV-Ab. The present study aims at assessing the diversity of infective and non-infective causes responsible for hepatitis with or without jaundice in AFH.
Subjects and Methods
Study design, period and setting
A hospital-based cross-sectional study was conducted at AFH, a tertiary hospital in Alexandria governorate, Egypt. The Liver Investigations unit (LIU) was responsible for setting the protocol of investigations and management. The study took place over a period of 12 months from January to December 2016.
Study sample
Admitted to AFH, a total of 111 patients were prospectively enrolled in the study, with the following inclusion criteria: acute hepatitis, chronic hepatitis, chronic liver disease, or liver cirrhotic patients suffering from jaundice with negative markers for hepatitis A, B and C.
Exclusion criteria were Patients who are jaundiced with positive markers for hepatitis A, B and C.
Data collection
a) A pre-tested data collection form was designed to collect and write down the following: Demographic characteristics (age, gender, race, address). History taken included (drug and alcohol consumption, previous pregnancy and operations). The state of patients upon admission (primary diagnosis, associated comorbidities), results of laboratory and immunological workup and clinical data details were recorded.
b) Clinical Data: After inclusion in the study, clinical data were obtained for all patients.
C) Laboratory and Immunological workup: Laboratory data were obtained for all patients at the selected time points. They included: Criteria of acute patients:
1) High serum Bilirubin.
2) ALT & AST > 3folds.
3) Negative viral markers (A, B, C). HAV-IgM, HBs-Ag, HCV-Ab
4) No signs of biliary obstruction in abdominal U/S.
The following acute patient investigations protocol was applied by the LIU [3-10]. It started by measuring the levels of the following immunoglobulins and biomarkers including:
1) HEV-IgM
2) HBc-IgM (Ab)
3) Leptospira-IgM
4) Non-hepatotropic viral markers: CMV-IgM, EB-IgM, Herpes S. –IgM
5) Autoimmune markers: ANA, ASMA, Anti-Actin, LKM-AB, AMA, Ig-G, Ig-M, ANCA
If all found to be negative the following investigations were proceeded:
6) PCR for HCV (Acute HCV)
7) If +ve HBc-IgM -à PCR for HBV
If these two were negative the following investigations were proceeded:
8) Metabolic markers: S. Iron, Ferritin, transferrin saturation, ceruloplasmin, urine copper, a1-antitrepsin [11-15]
Criteria of chronic patients
Undiagnosed jaundice, undiagnosed chronic liver disease, or liver cirrhosis with negative markers for hepatitis A, B or C. Similarly, the following chronic patient investigations protocol was applied by the LIU. It started by measuring the levels of the following immunoglobulins and biomarkers including:
1) Autoimmune markers: ANA, ASMA, Anti-Actin, LKM-AB, AMA, Ig-G, Ig-M, ANCA
2) Metabolic markers: S. Iron, Ferritin, transferrin saturation, ceruloplasmin, urine copper, a1-antitrepsin
3) HBc-IgM (Ab) – (Occult HBV) -> PCR for HBV
If all found to be negative the following investigations were proceeded:
4) Liver biopsy
5) Other investigations: CT, Triphasic CT, MRI, MRCP.
Statistical analysis
Collected data were entered and analyzed using the Statistical Package of Social Science Software (SPSS) program, version 21.0 IBM. Data were summarized using tables and graphs for quantitative and qualitative variables. Comparison between groups was performed using Chi square tests. P values below 0.05 were considered statistically significant.
Results
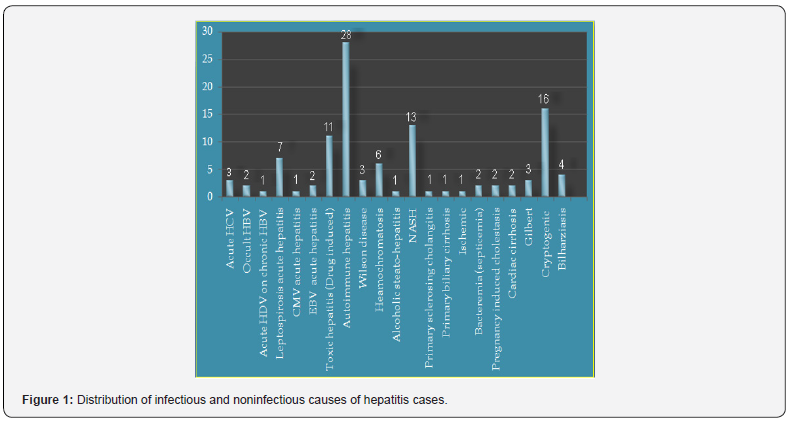
The distribution of hepatitis causes among the study participants revealed that the most common cause was autoimmune hepatitis 25.2% followed by cryptogenic (14.4%) and NASH (11.7%) (Figure 1). Gender distribution of the study participants showed that 56.8 % were females and only 43.2% were males. All patients (100%) who suffered from bacterial (septicemia, liver abscess), cardiac cirrhosis, pregnancy induced cholestasis, primary biliary cirrhosis, primary sclerosing cholangitis, Wilson disease were females while all patients (100%) who suffered from acute HDV on chronic HBV, alcoholic steato-hepatitis, CMV acute hepatitis and Ischemic hepatitis were males. Most patients with toxic hepatitis (Drug induced) (81.8%), autoimmune hepatitis (72.4%) acute HCV (66.7%) were females, while most cases with leptospirosis (85.7%), hemochromatosis (83.3%), Bilharziasis (75%) were males. The differences between males and female’s patients’ distribution over various liver diseases were statistically significant (P <0.05) (Table 1).
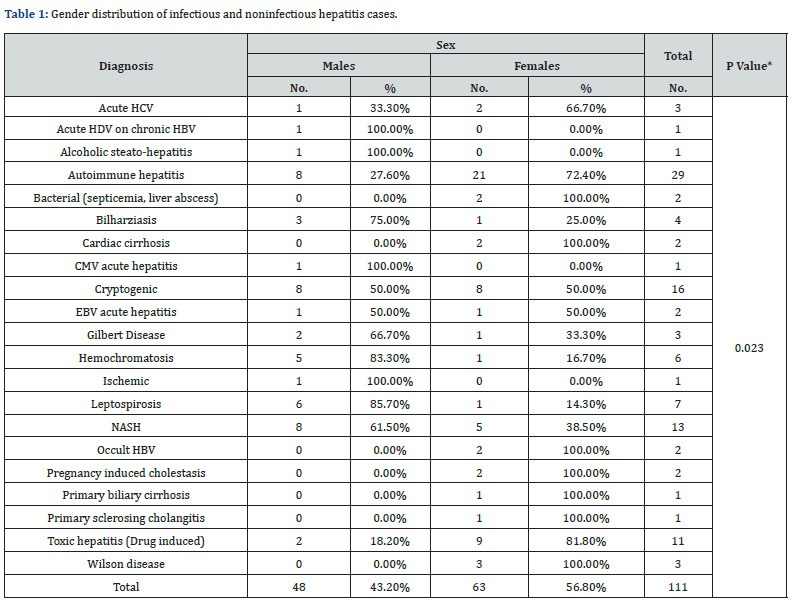
*P-value: Statistically Significant
In the (20-29) age group, Hepatitis patients mostly suffered from Wilson disease (50%) followed by pregnancy induced cholestasis (33.3%). In the (30-39) age group, patients suffered from leptospirosis (38.9%) followed by acute HCV and Gilbert disease both formed (16.7%). In the (40-49) age group, patients suffered from autoimmune hepatitis (42.6%) followed by cryptogenic type (23.5%). In the (50-59) age group, most patients suffered from NASH (76.5%). 60 years old and above, all patients suffered only from bacteria (septicemia, liver abscess). The overall differences between the five age groups were statistically significant (P < 0.001) (Table 2).
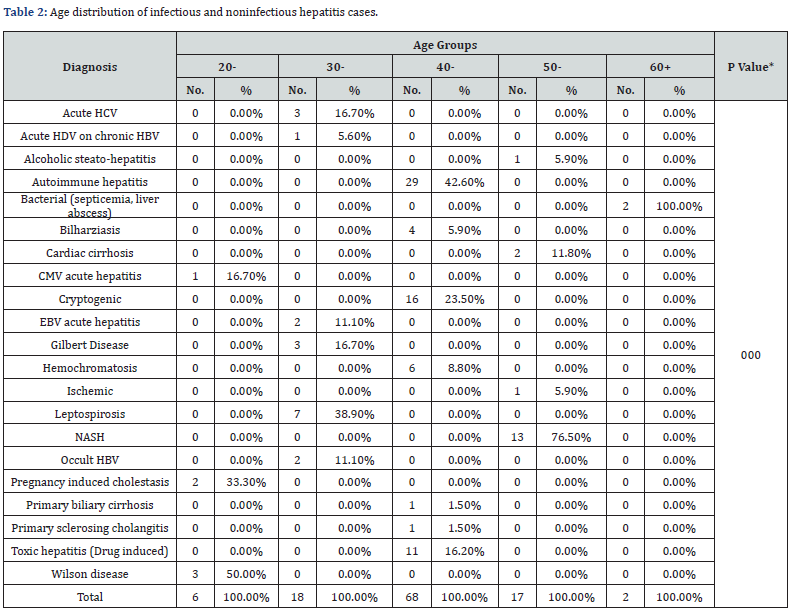
*P-value: Statistically Significant
Discussion
The current study demonstrated the variety of infective and non-infective causes of hepatitis with or without jaundice. In addition, it demonstrated its novel aspect in exploring age and gender distribution among fever hospital patients. The study revealed that the most common cause of acute hepatitis was autoimmune hepatitis; it was more common in females (72.4%) in the (40-49) age group. There is apparent discrepancy between this finding and literature that confirms that autoimmune hepatitis occurs more often in women in the second and third decades of life [1]. This variation can be attributed to the relatively small sample size.
The current study revealed that in acute jaundice or hepatitis patients, toxic hepatitis came as the second cause followed by leptospiral, CMV, EBV, acute HCV, acute HDV on top of HBV, bacterial, then Wilson disease. Like paracetamol overdose that is possibly the commonest cause of acute liver failure (ALF) in western countries like UK and USA, toxic hepatitis (Drug induced hepatitis) is rarely seen in developing countries like Egypt. Among Egyptians, most common drug induced hepatitis are antibiotics (most commonly rifampicin), oral contraceptives and Non Steriodal Anti-Inflammatory Drugs (NSAIDs) [16]. Interestingly, leptospirosis came in third place. This goes in agreement with several studies that demonstrated that clinical presentation like (ALF) is a complication of many tropical infections, and other conditions may mimic ALF, but may have minimal differences from ALF. In some common infections as dengue fever, leptospirosis, amoebic liver abscesses and other bacterial and fungal infections, liver failure may not be the underlying pathophysiology. Jaundice and encephalopathy dominate the clinical presentation of such infections. Other conditions can mimic ALF because clinical presentations in these conditions are also dominated by jaundice and encephalopathy including pregnancy related liver diseases [17]. In the current study, pregnancy induced cholestasis and represented (1.8%) of the study participants. Similarly, EBV acute hepatitis represented (1.8%) of the study participants. This goes in accordance with another study where (0.85%) had EBV hepatitis. Most of them presented with clinical/ biochemical evidence of jaundice. Patients with EBV hepatitis were presented with the classical features of infectious mononucleosis (fever, sore throat and lymphadenopathy). Splenomegaly was present in (88%) of patients. Ten of 17 (59%) patients were aged >30 years, and seven of 17 (41%) patients were aged ≥60 years [18].
Also, this study demonstrated that acute HCV (2.7%) was the most common hepatotropic viral infection. With more than one virus, Co-infections were only present in single patient in the form of acute HDV on chronic HBV. In comparison with other studies such as the one done in rural North India, food borne HAV (26.96%) was identified as the most common cause of acute hepatitis followed by HEV (17.97%), HCV (11.98%). With more than one virus, Co-infections were present in the form of HAV-HEV co-infection being the most common [19]. These differences in results were attributed to differences in sample size in addition to differences in population characteristics. While food borne infections are most common in underserved rural population in India, Alexandria is an urban city with more sanitary environmental facilities including safe water supply and sanitary sewage disposal. This minimizes percents of food borne infections transmission.
In agreement with the results of the current study, a study was done in an endemic area among 110 Egyptian patients with acute jaundice. The majority were diagnosed having communityacquired acute HCV hepatitis. Apart from ALF, sub-acute hepatic failure (SAHF) as well as acute-on-chronic liver failure (ACLF) due to viral hepatitis are commonly diagnosed among Egyptians. Acute HCV progresses to chronic infections in 55-85% of cases [20]. HCV is highly endemic in Egypt and, according to Egyptian Demographic and Health Survey (2008), estimated prevalence was 14.7% among (15-59) age group. With 10% of its population being chronically infected, Egypt has the highest prevalence of HCV in the world. In Egypt, the HCV epidemic is thought to have originated from unsafe injections administered for mass antischistosomiasis campaigns conducted during 1960s and 1970s. Currently, contact with infected blood including unsafe injection practices in health care settings is considered the primary mode of HCV transmission in Egypt.
In the present study, Wilson disease (WD), represented (2.8%), from patients and all lied in the 20-29 age group. This is similar to another study assessed by the European Association for the Study of the Liver (EASL) guidelines. Wilson disease (WD) patients with acute hepatic phenotype were younger than 30 years and had survived an acute episode of hemolytic anemia with residual liver disease of cirrhosis or chronic hepatitis. In chronic jaundice or hepatitis patients, NASH was the commonest cause followed by metabolic causes, hemochromatosis, cardiac cirrhosis, then alcoholic cirrhosis. In a study of Ravi S et al. [21] of 607 patients, 62 % suffered from nonalcoholic steatohepatitis (NASH). There were no differences for age, gender, BMI, cirrhosis at presentation. Their mean age was 50±15 year; 52 % males.
In Pakistani a study 2015, a total of 22 patients were identified as having hemochromatosis. All subjects were men with a mean age of 53±9.2 years at the time of diagnosis. The most common presentation was skin pigmentation seen in 17 (77%) Patients with diabetes who were diagnosed earlier as compared to those without it. Eighteen (81%) subjects had cirrhosis at the time of diagnosis [22].
Conclusion
In our study, we investigated the etiology amongst inpatients and liver clinic outpatients to try to capture the diversity of infective and non-infective causes responsible for jaundice or hepatitis of A, B, C virologically negative patients in Alexandria city. In acute jaundice or hepatitis patients, autoimmune hepatitis was the commonest cause followed by toxic hepatitis, leptospiral, CMV, EBV, acute HCV, acute HBV or acute HDV on top of HBV, bacterial, then Wilson disease. In chronic jaundice or hepatitis patients, NASH was the most common cause followed by metabolic causes, hemochromatosis, cardiac cirrhosis, then alcoholic cirrhosis.
Limitation to the Study
Generalization of the findings of this study is hindered by the relatively small sample size.
Ethical Disclosures
Protection of human subjects: The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee. Informed consent was obtained directly from each patient before data collection and after explanation of the study objectives. All procedures of data collection were treated with confidentiality according to the Helsinki declarations of biomedical ethics.
Acknowledgments
The authors are thankful to the Chairmen of the AFH for conducting this study. The authors also thank all the nursing staff who helped the researchers during the data collection process. The research team also thanked all the patients.
References
- PC Hayes, KJ Simpson, OJ Garden (2010) Liver and biliary tract diseases. DAVIDSON'S Principles and Practice of Medicine Elsevier Science Limited, Churchill Livingstone, USA, p. 1103-1209.
- Bounlu K, Insisiengmay S, Vanthanouvong K, Saykham Widjaja S, Iinuma K (1998) Acute jaundice in Vientiane, Lao People's Democratic Republic. Clin Infect Dis. 27(4): 717-721.
- Roque-Afonso AM, Grangeot-Keros L, Roquebert B, Desbois D, Poveda JD, et al. (2004) Diagnostic relevance of immunoglobulin G avidity for hepatitis A virus. J Clin Microbiol 42(11): 5121-5124.
- Roque-Afonso AM, Mackiewicz V, Dussaix E (2006) Detection of immunoglobulin M antibody to hepatitis A virus in patients without acute hepatitis A: the usefulness of specific immunoglobulin G avidity. Clin Infect Dis 42: 887-888.
- Seriwatana J, Shrestha MP, Scott RM, Tsarev SA, Vaughn DW, et al. (2002) Clinical and epidemiological prevalence of quantitating hepatitis E virus-specific immunoglobulin M. Clin Diagn Lab Immunol 9(5): 1072-1078.
- Innis BL, Seriwatana J, Robinson RA, Shrestha MP, Yarbough PO, et al. (2002) Quantitation of immunoglobulin to hepatitis E virus by enzyme immunoassay. Clin Diagn Lab Immunol 9: 639-648.
- Tsarev SA, Emerson SU, Tsareva TS, Yarbough PO, Lewis M, et al. (1993) Variation in course of hepatitis E in experimentally infected cynomolgus monkeys. J Infect Dis 167(6): 1302-1306.
- Pybus OG, Barnes E, Taggart R, Lemey P, Markov PV, et al. (2009) The Genetic history of the hepatitis C virus in East Asia. J Virol 83: 1071-1082.
- Newton PN, Rolain JM, Rasachak B, Mayxay M, Vathanatham K, et al. (2009) Sennetsu neorickettsiosis - a probably fish borne cause of fever rediscovered in Laos. Am J Trop Med Hyg 81: 190-194.
- Cole JR, Sulzer CR, Pursell AR (1973) Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol 25(6): 976-980.
- Maheshwari A, Ray S, Paul J, Thuluvath PJ (2008) Acute hepatitis C. Lancet 372(9635): 321-332.
- Blacksell SB, Myint KSA, Khounsy S, Phruaravanh M, Mammen MP, et al. (2006) Prevalence of hepatitis E virus antibodies in pigs: Implications for human infections in village-based subsistence pig farming in the Lao PDR. Trans R Soc Trop Med Hyg 101(3): 305-307.
- Songsivilai S, Jinathongthai S, Wongsena W, Tiangpitayakorn C, Dharakul T (1997) High prevalence of hepatitis C infection among blood donors in northeastern Thailand. Am J Trop Med Hyg 57(1): 66-69.
- Corwin AL, Dai TC, Duc DD, Suu PI, Van NT, et al. (1996) Acute viral hepatitis in Hanoi. Trans R Soc Trop Med Hyg 90(6): 647-648.
- Thaipadungpanit J, Wuthiekanun V, Chierakul W, Smythe LD, Petkanchanapong W, et al. (2007) Dominant clone of Leptospira interrogans associated with an outbreak of human leptospirosis in Thailand. PLoS Negl Trop Dis 1(1): e56.
- Anand AC, Garg HK (2015) Approach to clinical syndrome of jaundice and encephalopathy in tropics. J Clin Exp Hepatol 5(Suppl 1): S116-130.
- Vine LJ, Shepherd K, Hunter JG, Madden R, Thornton C, et al. (2012) Characteristics of Epstein-Barr virus hepatitis among patients with jaundice or acute hepatitis. Aliment Pharmacol Ther 36(1): 16-21.
- Jain P, Prakash S, Gupta S, Singh KP, Shrivastava S, et al. (2013) Prevalence of hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis D virus and hepatitis E virus as causes of acute viral hepatitis in North India: a hospital-based study. Indian J Med Microbiol 31(3): 261-265.
- Quinti I, el-Salman D, Monier MK, Hackbart BG, Darwish MS, et al. (1997) HCV infection in Egyptian patients with acute hepatitis. Dig Dis Sci 42(10): 2017-2023.
- Hayashi H, Tatsumi Y, Yahata S, Hayashi H, Momose K, et al. (2015) Acute Hepatic Phenotype of Wilson Disease: Clinical Features of Acute Episodes and Chronic Lesions Remaining in Survivors. J Clin Transl Hepatol 3(4): 239-245.
- Ravi S, Shoreibah M, Raff E, Bloomer J, Kakati D, et al. (2015) Autoimmune Markers Do Not Impact Clinical Presentation or Natural History of Steatohepatitis-Related Liver Disease. Dig Dis Sci 60(12): 3788-3793.
- Parkash O, Akram M (2015) Hereditary Hemochromatosis. J Coll Physicians Surg Pak 25(9): 644-647.






























