Secretory Inhibitor of Lysozyme and Biofilm Formation of intestinal Strains Candida in Childrens with Reactive Arthritis
Oleg V Bukharin1, Natalia B Perunova1*, Elena V Ivanova1, Olga E Chelpachenko1 and Anatoly I Khavkin2
1Institute of Cellular and Intracellular Symbiosis, Ural Department, Russian Academy of Science, Laboratory of Biomonitoring and Molecular-Genetic Research, Orenburg, Russia
2Academician Yu. E. Veltishchev Research and Clinical Institute of Pediatrics at the N. I. Pirogov Russian National Research Medical University, Moscow, Russia
Submission:September 09, 2021; Published:April 03, 2023
*Corresponding author:Natalia B. Perunova, Institute of Cellular and Intracellular Symbiosis, Ural Department, Russian Academy of Science, Laboratory of Biomonitoring and Molecular-Genetic Research, Orenburg, Russia
How to cite this article:Oleg V Bukharin1, Natalia B Perunova, Elena V Ivanova, Olga E Chelpachenko and Anatoly I Khavkin. Secretory Inhibitor of Lysozyme and Biofilm Formation of intestinal Strains Candida in Childrens with Reactive Arthritis. Adv Res Gastroentero Hepatol, 2023; 19(3): 556013. DOI: 10.19080/ARGH.2023.19.556013.
Abstract
This short communication compares the phenotypic properties of Candida species isolated from gut of children with reactive arthritis, such as secretory inhibitor of lysozyme and biofilm formation. A total of 65 clinical strains of Candida species isolated from the feces of healthy children and children’s with ReA were tested. SIL production of Candida by inhibiting lysozyme activity against Micrococcus luteus ATCC 15307 were studied (mg/mL*OD). Quantitative assessment of biofilm formation was performed using crystal violet binding assay method (CU). A significantly higher proportion of ReA strains were SIL-positive (77.8% vs. 40.0%) and BF-positive (64.4% vs. 30.0%) compared with non-ReA strains (P<0.01). These results suggest that the functional significance of SIL production and biofilm formation must be estimated, of investigating the roles of intestinal of Candida in the pathogenesis of spondylarthritis, including reactive arthritis. It is imperative to delineate microbial factors that contribute to ReA development.
Keywords: Candida albicans; Phenotypic properties; Reactive arthritis
Introduction
At this time, reactive arthritis (ReA) refers to an infection gastrointestinal tract induced systemic illness, characterized by a sterile synovitis occurring in a genetically predisposed individual [1,2]. Numerous experimental studies have shown that the gut microbiota is an important pathogenetic factor in the development of spondylarthritis, including reactive arthritis. The connection of arthritis with dysbiotic gut microbiota disorders has been proved [3,4]. A number of authors point to the role of fungi of the gut microbiota, and their phenotypic properties in the development of inflammatory diseases of the intestinal and extra-intestinal localization [5,6]. It has been ReA subjects suffer from intestinal dysbiosis characterized by an abnormal expansion of the Candida population [7]. Candida albicans is a gut commensal and opportunistic pathogen. Among virulence factors of C. albicans are extracellular hydrolytic enzymes (lipases, phospholipases and secreted aspartyl proteinases), adherence and pleomorphism [8]. Research results demonstrate that biofilm production could enhance the invasiveness and virulence of strains C. albicans [9]. In addition, a study by Deckers et al. [10] showed importance of the lysozyme inhibitors promote microorganisms in growth or survival in the ecological niches in the host. The results of other authors [11,12] suggest that the inactivation of antimicrobial proteins (lysozyme, lactoferrin, defensins) may be important for commensal and pathogens to induce chronic inflammatory diseases of the human body. Thus, the aim of this work was to compare phenotypic properties of Candida species associated with the source of ReA, such as secretory inhibitor of lysozyme (SIL) and biofilm formation (BF).
Methods. This study included 109 childrens ranging in age from 5 to 16years. Not all specimens yielded some fungi growth. A total of 65 non‐duplicate, clinical isolates of Candida species, isolated from the feces of healthy children (n=20) and children with ReA (n=45). Forming of the investigated groups of children was conducted on the base of child’s hospital № 6, Orenburg. On admission to the hospital the legal representatives of patient (mother, father, guardian) were acquainted and signed in a document about the informed voluntarily consent to medical interference that included a voluntarily consent to realization of necessary clinical and laboratory methods of research, including bacteriological and immunological methods (head 4 Federal laws from 21.11.2011г. № 323-FL “About bases of health of citizens care in Russian Federation”). For the diagnosis of ReA, the criteria approved by the International RA Meeting (Berlin,1996, 1999) was used.
Stool samples from childrens were homogenized in sterile 0.9% normal saline and cultured on Sabouraud glucose agar (2% D-glucose), incubated aerobically at 26°C for 3–5 days and the values of log 10cfu/g were calculated. All fungal isolates grown on the selective medium were isolated to obtain single-cell pure colonies. Identification of Candida spp was with the use of the test system API20CAUX (bioMerieux, France). For the fungi to verify identification MALDI-TOF mass-spectrometr «Microflex» («Bruker Daltonics», Germany) was used.
The strains were assessed to SIL production used photometric method Bukharin OV et al. The biomass of the cultures to be tested for production of SIL was seeded with a standard bacteriological loop in 3ml of liquid nutrient medium (Luria Bertani Broth, HiMedia) containing standard lysozyme solution (20μg/ mL) (Sigma Chemical Co., St Louis, MO, USA). After incubation for 24h at 26°C, then the optical density (OD) of broth culture against broth (Y) was measured on the elx808 photometer (BioTek, USA), wavelength 450nm. The supernatant was separated from microbial cells by centrifugation at 3000rpm for 15minutes. As a test strain to determine of SIL used acetonized culture of Micrococcus luteus ATCC 15307. The culture was dissolved in a physiological solution, the optical density (OD) of the test strain was adjusted to 0.30 (0.28-0.32) on the elx808 spectrophotometer (BioTek, USA), wavelength 450nm. Then 50μl of the supernatant-lysozyme mixture was placed in a polystyrene plate in vertical rows and the wells of each row of the tablet were filled with 200 μl suspension of Micrococcus by multichannel pipette. SIL production of each strain was indicated as according to the degree of lysis of the suspension and was expressed in μg/mL of inactivated lysozyme (mcg/ml*OD). SIL production of strain up than 0,50mcg/ml*OD corresponded was SIL-positive strains.
Quantitative assessment of biofilm formation of Candida was performed using crystal violet binding assay method [13]. Briefly, strains were grown in Luria-Bertani broth for 48h in ELISA plate together with negative control (the nutrient broth); growth was verified by measuring optical density (OD) with elx808 spectrophotometer. The number of inoculated plankton cells was calculated on elx808 spectrophotometer (BioTek, USA), wavelength 540nm and expressed in conventional optical density units (OD). The analyses were performed in triplicates and the median value was used for analysis. After 48hours, plankton cells of microorganisms were removed from the holes of the panel and biofilms were stained. To do this, 150μl of distilled water and 20μl of 1% crystal violet were gently (without stirring) introduced into the hole and incubated for 45min at room temperature. After a thorough three-time washing with distilled water into the wells for the extraction of dye from the films were added 200mcl of 96% ethanol and measured the optical density of this solution at a wavelength of 630nm. The intensity of staining the contents of the holes corresponded to the quantity of BF. The ability to BF was expressed in units. Degree of biofilm formation was presented in conditional units (CU) which was the optical density of the broth after growth of the strain relative to the nutrient broth optical density (negative control). BF production of strain up than 1,0 CU corresponded was BF-positive strains.
The data are presented as the mean±SD. The Wilcoxon signed-rank test was used to assess the statistical significance of difference between control and the samples. A value of p <0.05 was considered statistically significant.
Results. Сandida species were isolated more frequently from ReA group (93,8±6,5%) than from control group (32,8±2,6%) while C. lusitanie, C. tropicalis and C. parapsilosis were found only in specimens obtained from children with arthritis. The isolation of C. albicans and C. krusei was characteristic of both groups. In healthy children yeast was represented by the species C. albicans (75,0±3,3%) and C. krusei (25,0±1,1%). Among the Candida species in children with ReA, the species C. albicans dominated, which accounted for 55,6±3,7% of the total number of cultures. Other species included C. krusei - 17,8+1,2%; C. lusitanie - 11,1±0,7%; C. tropicalis 11,1±0,7 % and C. parapsilosis – 4,4±0,3%. The concentration of microorganisms from control group were 3,1±0,3 log- 10cfu/g versus 6,3±0.5log 10cfu/g from ReA group.
Of the ReA strains tested, 35/45 (77.8%) were considered to be SIL-positive (Table 1) compared with only 8/20 of the non- ReA isolates (40.0%, P< 0.05). Furthermore, a significantly higher proportion of ReA strains (64.0 % vs. 30.0%) were BF-positive compared with non-ReA strains (P<0.01). In contrast to the fungi isolated from the control group (Table 2), strains isolated from children’s with ReA showed more intensive inhibition of the fungicidal activity of lysozyme (1,35±0,1 mcg/ml*OD vs. 0,71±0,05 mcg/ml*OD, P < 0.05).
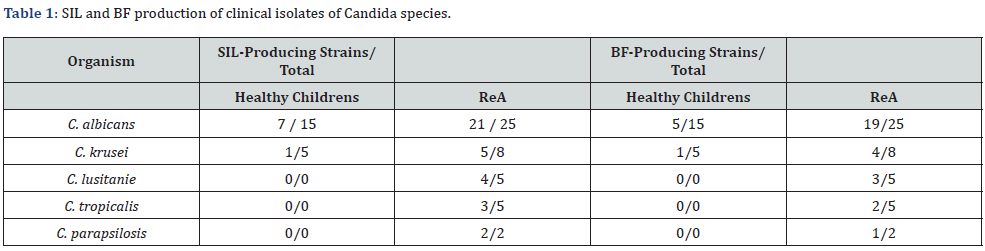
SIL: Secretory Inhibitor of Lysozyme; BF: Biofilms Formation; ReA: Chronic Reactive Arthritis
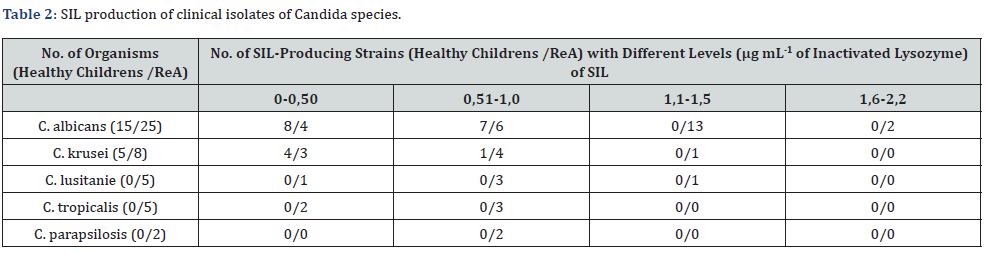
SIL: Secretory Inhibitor of Lysozyme; ReA: Chronic Reactive Arthritis
Among the BF-producing strains, fungi isolated from patients without ReA had mean BF production levels of 1,5±0,02 CU. The cultures of Candida from the ReA group were more active in biofilms formation (4,8±0,9 CU, P < 0.05). C. albicans strains ReA group were more potent biofilm producers than strains non-ReA group (7,7±0,5 CU vs. 1,5±0,05 CU, P=0.048). In comparison with this, other types of yeast isolates from the ReA group showed a low activity of formation biofilm (C. krusei – 4,5±1,2 СU; C. lusitanie – 3,1±0,7 CU; C. tropicalis 2,1±0,7 CU and C. parapsilosis – 3,4±0,3CU, respectively).
Discussion. Interaction between an arthritogenic agent and a predisposed host is the basis for development of ReA [1]. Currently, changes in the intestinal flora and local changes in the balance between of pro- and anti-inflammatory factors of immunity are considered as a trigger for reactive arthritis [2,3]. Opportunistic bacteria and fungi counteract the host immune defense by excreting various inhibitors. The inactivation of components of innate immunity (lysozyme) may be important for opportunistic pathogens to avoid clearance by microbicidal proteins and persistent surviving [5,11,12]. Biofilm is an important virulence factor Candida and also persistent surviving. Biofilm formation provides Candida species with the ability to evade host immune defenses and resistance to antifungals [14].
In this work, we showed that Candida strains from the feces in the ReA group have high values of biofilm formation and lysozyme inhibition (anti-lysozyme potential). These properties can be important in the survival of fungi and therefore can be influenced to prevent dysbiosis. Previously shown that gut dysbiosis (Сandida yeast overgrowth) may be triggers of arthritis. It is obvious that SIL and BF also are markers of persistence of Candida, which colonizes the intestine in patients with RA. In this regard, we assume that the influence (reduction) of biofilm formation and inhibition of lysozyme can help achieve positive results in the prevention and treatment of ReA.
Funding
This work was carried out in the framework of fundamental research at the Ural Branch of the Russian Academy of Sciences (project 18-7-8-34).
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Authors Contributions
Oleg Bukharin: Conceptualization, Methodology.
Natalya Perunova: Data curation, Writing- Original draft preparation.
Elena Ivanova: Visualization, Investigation.
Olga Chelpachenko: Writing - Reviewing and Editing.
Conflicts of Interest/Competing Interests
All authors listed on the title page are aware of this submission and all contributions are attributed in the author list or acknowledgment section.
Ethics Approval
On admission to the hospital, the legal representatives (mother, father and guardian) of the patient were acquainted and signed a document about informed voluntary consent to medical interference, which included a voluntary consent to the realization of necessary clinical and laboratory research methods, including bacteriological and immunological methods (Head 4 of Federal Law from 21.11.2011 No. 323-FL “About bases of health of citizens care in Russian Federation”).
Consent to Participate
On admission to the hospital, the legal representatives (mother, father and guardian) of the patient were acquainted and signed a document about informed voluntary consent to participate for research.
Consent for Publication
All authors read and approved the final manuscript.
References
- Stavropoulos P, Soura E, Kanelleas A, Katsambas A, Antoniou C, et al. (2015) Reactive arthritis. J Eur Acad Dermatology Venereol 29(3): 415-424.
- García-Kutzbach A, Chacón-Súchite J, García-Ferrer H, Iraheta I (2018) Reactive arthritis: update. Clin Rheumatol 37(4): 869-874.
- Yeoh N, Burton JP, Suppiah P, Gregor Reid, Simon Stebbings (2013) The role of the Microbiome in Rheumatic Diseases. Curr Rheumatol 15(3): 314.
- Sekirov I, Russell SL, Antunes CM, Finlay BB (2010) Gut Microbiota in Health and Disease. Physiol Rev 15(3): 314.
- Bukharin OV, Perunova NB (2014) Microsymbiocenosis. Ekaterinburg: UrDepart RAS.
- Sonoyama K, Miki A, Sugita R, Haruka Goto, Mayumi Nakata, et al. (2011) Gut colonization by Candida albicans aggravates inflammation in the gut and extra-gut tissues in mice. Med Mycol 49(3): 237-247.
- Chelpachenko OE, Bukharin OV, Danilova EI, Fedotova LP, Perunova NB, et al. (2017) A clinical case of Candida arthritis in a child. Clinical Practice in Pediatrics.
- Höfs S, Mogavero S, Hube B (2016) Interaction of Candida albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota. J Microbiol 54(3): 149-169.
- Borghi E, Romagnoli S, Fuchs BB, Daniela Cirasola, Federica Perdoni, et al. (2014) Correlation between Candida albicans biofilm formation and invasion of the invertebrate host Galleria mellonella. Future Microbiol 9(2): 163-173.
- Deckers D, Vanlint D, Callewaert L, Aertsen A, Michiels CW (2008) Role of the lysozyme inhibitor Ivy in growth or survival of Escherichia coli and Pseudomonas aeruginosa bacteria in hen egg white and in human saliva and breast milk. Appl Environ Microbiol 74(14): 4434-4439.
- Bukharin OV (1999) Persistence of pathogenic bacteria. Medicine, Moscow.
- Ivanov IB, Gritsenko VA, Kuzmin MD (2009) Phenotypic differences between coryneform bacteria isolated from seminal fluid of healthy men and men with chronic prostatitis syndrome. Asian J Androl 11(4): 517-520.
- O'Toole GA, Kolter R (1998) Initiation of biofilm formation in Pseudomonas fluorescens WCS365 processes via multiple, convergent signaling paths: a genetic analysis. Mol Microbiol 28(3): 449-461.
- Dantas AS, Lee KK, Raziunaite I, Katja Schaefer, Jeanette Wagener, et al. (2016) Cell biology of Candida albicans – host interactios. Curr Opin Microbiol 34: 111-118.






























