Piperacillin-Tazobactam Versus Piperacillin-Tazobactam Plus Rifaximin for the Treatment of Spontaneous Bacterial Peritonitis in Patients with Liver Cirrhosis: A Prospective Comparative Open-Label Interventional Study
Pukar Thapa*, Sudhamshu KC, Binod Karki, Niyanta Karki, Sital Karki and Pooja Thapaliya
Internal Medicine, Hepatology, National Academy of Medical Sciences, Nepal
Submission:November 11, 2022; Published:November 28, 2022
*Corresponding author:Pukar Thapa, Assistant Professor, Internal Medicine, Hepatology, National Academy of Medical Sciences, Kathmandu, Nepal
How to cite this article:Pukar T, Sudhamshu K, Binod K, Niyanta K, Sital K, et al. Piperacillin-Tazobactam Versus Piperacillin-Tazobactam Plus Rifaximin for the Treatment of Spontaneous Bacterial Peritonitis in Patients with Liver Cirrhosis: A Prospective Comparative Open-Label Interventional Study. Adv Res Gastroentero Hepatol, 2022; 19(1): 556004. DOI: 10.19080/ARGH.2022.19.556004.
Abstract
Background: Cirrhosis represents a late stage of progressive hepatic fibrosis with an increased risk of numerous complications and a decreased life expectancy. Spontaneous bacterial peritonitis (SBP) is the most common bacterial infection in cirrhosis patients. It is necessary to recognize SBP early during illness to ensure a good outcome due to a short window period to intervene. Piperacillin- tazobactam is a broad-spectrum antibiotic that has activity against many Gram-positive and Gram- negative bacteria. Rifaximin is a virtually unabsorbable antibiotic with a broad-spectrum activity against gram-positive and gram-negative microorganisms within the gastrointestinal tract. It minimizes antimicrobial resistance and adverse events and renders the drug safe in all patients. Intestinal decontamination with rifaximin is an attractive approach for the treatment of patients with cirrhosis with SBP.
Materials and Methods: This research was a hospital-based prospective comparative open-label interventional study carried out in the Liver unit of one of the tertiary hospitals in Nepal from July 2019 to June 2020 for the total duration of 1 year. Patients of cirrhosis who were diagnosed with SBP were included. Patients with hepatic encephalopathy, renal impairment (creatinine >3mg/dl), allergy to drugs, already on SBP/HE prophylaxis, presence of HCC were excluded. Diagnostic aspiration of the ascitic fluid of all admitted patients was performed at the time of admission. The patients were allocated into two equal groups randomly. Patients in group 1 received Piperacillin-tazobactam 4.5gm 8 hourly intravenously for five days and Rifaximin 1100mg/day orally in two divided doses for five days. Patients in group 2 received Piperacillin-tazobactam 4.5gm 8 hourly intravenously for five days. The included patients underwent repeat diagnostic aspiration and blood sample tests on the third day of antibiotic therapy to compare the change of ascitic fluid WBC, PMN cells and peripheral WBC after 48 hours of treatment in both groups. A proforma was used to collect the data from the patients enrolled in the study. Data were entered and analyzed using SPSS version 26 software.
Results: A total of 34 patients were enrolled, 17 in each group. Out of 34 patients, 76% were male whereas the mean age of the participants was 51.65(±10.21) years. There was male predominance (76%). Ascitic fluid WBC in group 1 was 1891±988 cells/mm3 on the first day and was 84.7±83.5 cells/mm3 on the third day of the therapy. Reduction in the ascitic fluid WBC was statistically significant in both groups with a p-value of less than 0.001. Moreover, the reduction in ascitic fluid WBC count on the third day was statistically significant in group 1 compared with group 2 with a p-value of 0.02. Ascitic fluid PMN, serum creatinine, and peripheral WBCs were reduced significantly in both groups. Group 1 had a clinically significant reduction (p=0.03, 0.02, and 0.001 respectively) of these parameters on the third day than in group 2.
Conclusion: In our study, rifaximin plus Piperacillin-tazobactam showed more favorable effects in reducing peripheral WBC, creatinine, ascitic fluid WBC, and duration of hospital stay in the treatment of SBP than Piperacillin-tazobactam only. However, the clinical effects and potential role of rifaximin and combination therapy in the management of SBP need further clarification in further studies due to the smaller number of patients in this study.
Keywords: Peripheral WBC; Creatinine; Ascitic fluid WBC; Piperacillin-tazobactam; Hepatic fibrosis
Abbreviations: HE: Hepatic Encephalopathy; PPH: Porto- Pulmonary Hypertension; HPS: Hepato-Pulmonary Syndrome; HCC: Hepatocellular Carcinoma; HRS; Hepatorenal Syndrome; HVPG: Hepatic Venous Pressure Gradient; UTI: Urinary Tract Infections; MDROs: Multiple Drug Resistant Organisms; BT: Bacterial Translocation; SIBO: Small Intestinal Bacterial Overgrowth; OPD: Outpatient Department; IRB: Institutional Review Board
Introduction
Cirrhosis represents a late stage of progressive hepatic fibrosis characterized by distortion of the hepatic architecture and the formation of regenerative nodules. Patients with cirrhosis are at increased risk of numerous complications and have a decreased life expectancy [1]. Some of the major complications of cirrhosis include varices, ascites, hepatic encephalopathy (HE), portopulmonary hypertension (PPH), hepato-pulmonary syndrome (HPS), and hepatocellular carcinoma (HCC), hepatorenal syndrome (HRS), SBP, and coagulation disorders. Portal hypertension can lead to the formation of venous collaterals, biochemical (increased production of vasoconstrictors, vascular endothelial growth factor, nitric oxide, and other splanchnic vasodilators) and functional abnormalities (plasma volume expansion and increased cardiac output), and thus contribute to the pathogenesis of many of the complications of cirrhosis. Hepatic venous pressure gradient (HVPG) measurement can help quantify portal hypertension. Portal hypertension is present when HVPG is > 5 but is clinically significant when > 10 [2].
Ascites is the most common complication/decompensating event of cirrhosis. It is also the most common complication that leads to hospital admission. Approximately 15% of the patients with ascites die in one year whereas 44% die in five years [3,4]. The worldwide prevalence of bacterial infection in hospitalized patients with cirrhosis ranges between 33% and 47% [5]. Prevalence of infection is related to the severity of liver disease and is more common in patients with Child C cirrhosis than Child A/ B cirrhosis. SBP is the most common infection in cirrhosis. Urinary tract infections (UTI), pneumonia, and bacteremia are responsible for 20%, 15%, and 12% respectively, infections in this patient population [6].
Spontaneous bacterial peritonitis is an ascitic fluid infection without an evident intra-abdominal surgically treatable source. The diagnosis of SBP is confirmed after obtaining a positive ascitic fluid bacterial culture, an elevated ascitic fluid absolute PMN of ≥250 cells/mm3, and exclusion of secondary causes of bacterial peritonitis. It is necessary to recognize SBP early in the course of infection because there is frequently a short window of opportunity to intervene to ensure a good outcome. Patients with SBP typically have advanced cirrhosis. The higher the MELD score, the higher the risk of SBP [7].
Empirical antibiotic therapy must be initiated immediately after the diagnosis of SBP. In the 1990s, cefotaxime, a thirdgeneration cephalosporin, was extensively investigated in patients with SBP because it covered most causative organisms and its ascitic fluid concentrations were high during therapy during that time [8]. However, the spread of resistant bacteria in the healthcare environment during the last two decades has led to an alarming increase in the number of infections caused by multiple drug resistant organisms (MDROs). Patients with advanced cirrhosis are highly susceptible to the development of infections caused by MDROs because of their repeated hospitalizations, regular exposure to invasive procedures, and frequent exposure to antibiotics, either as prophylaxis or as treatment. Bacterial resistance increases fourfold the risk of mortality of SBP [9]. Piperacillin/tazobactam has generally been preferred as the primary approach for community-acquired, health care, and nosocomial SBP in areas with a low prevalence of infections sustained by MDROs [10].
In patients who survive an episode of SBP, the cumulative recurrence rate at one year is approximately 70% [11]. Many patients receive rifaximin to prevent recurrent episodes of HE, which may also be effective against recurrent SBP [12]. Rifaximin is a broad-spectrum antibiotic that is effective against gram-positive and gram-negative microorganisms within the gastrointestinal tract. The main advantage of rifaximin is that it is virtually unabsorbable, which minimizes antimicrobial resistance and adverse events and renders the drug safe in all patient populations. In addition, rifaximin has better activity against gram-positive organisms than norfloxacin [13]. The reduction of endotoxemia by rifaximin may reduce bacterial translocation (BT) by causing a fall in portal pressures considering that portal hypertension induces structural abnormalities in intestinal mucosa leading to an enhanced permeability [14]. Overall, the effects of rifaximin on small intestinal bacterial overgrowth (SIBO) and BT are consistent with recent findings, showing a significantly reduced 5-year probability of SBP in cirrhotic patients taking rifaximin [15]. Moreover, rifaximin can promote the growth of beneficial gut bacteria; it actually does not change the overall composition of the gut flora, and the changes that occur are minimal [16]. Intestinal decontamination with rifaximin is an attractive approach in the treatment of patients with cirrhosis with SBP. Hence, this study was conducted to compare the efficacy of adding Rifaximin over Piperacillin-tazobactam with Piperacillin-Tazobactam alone in treating SBP with cirrhosis.
Materials and Methods
Study setting and design
This hospital-based prospective comparative open-label interventional study was conducted at the liver unit of the department of medicine at the National Academy of Medical Sciences (NAMS), Bir Hospital, one of the tertiary hospitals located in Kathmandu, Nepal.
Consecutive patients of liver cirrhosis aged 18 or above presenting to OPD (Outpatient Department) or ER (Emergency) from July 2019 to June 2020 with grade II/III ascites were enrolled in the study to assess for the presence of SBP. Diagnostic aspiration of the ascitic fluid was conducted in all admitted patients with cirrhosis at the bedside at the time of admission. Diagnosis of SBP was made according to IAC (2000) if the PMN cell count in the ascitic fluid exceeded 250/ml with no other sources of intraabdominal infection. Diagnosis of liver cirrhosis was upon clinical evaluation, liver function tests, and abdominal imaging with or without a liver biopsy.
Clinical evaluation for all patients was done, including complete medical history, clinical examination, and investigations that included liver and renal function tests, blood pictures, and coagulation profile. The included patients underwent repeat diagnostic aspiration and blood sample tests 48 hours of antibiotic therapy.
Patients with grade ≥ 3 hepatic encephalopathies, renal impairment (serum creatinine >3mg/dL), had a history of allergy to piperacillin-tazobactam or rifaximin, had recent exposure to other antibiotics, hepatocellular carcinoma, and who were unwilling to give consent, were excluded from the study.
Ethical clearance was taken from Institutional Review Board (IRB) of NAMS.
Intervention details
The patients with SBP were randomly divided into two equal groups. A lottery method was used where patients choosing lottery A were assigned to group 1 whereas the patients choosing lottery B were group 2. Patients in group 1 received Piperacillintazobactam 4.5gm 8 hourly intravenously for five days and Rifaximin 1100mg/day orally in two divided doses for five days. Patients in group 2 received Piperacillin-tazobactam 4.5gm 8 hourly intravenously for five days.
Data analysis and statistical analysis
The sample size calculation for Continuous Endpoint, two Independent Sample study was used and correcting it for finite population, the final sample size was 34 (17 in each group).
A proforma was used to collect the data from the interview and investigation records of patients enrolled in the study. The data from the Proforma were recorded in the SPSS software (Version 26). Quantitative data were taken from Mean, SD, median, and range. The student’s t-test was used to compare the means of the two groups. The means between the same groups were compared using paired sample t-test. A Pearson chi-square test was used for categorical data. Bivariate and multivariate analysis was done for predicting the outcome. A P-value that was less than 0.05 was considered significant.
Results
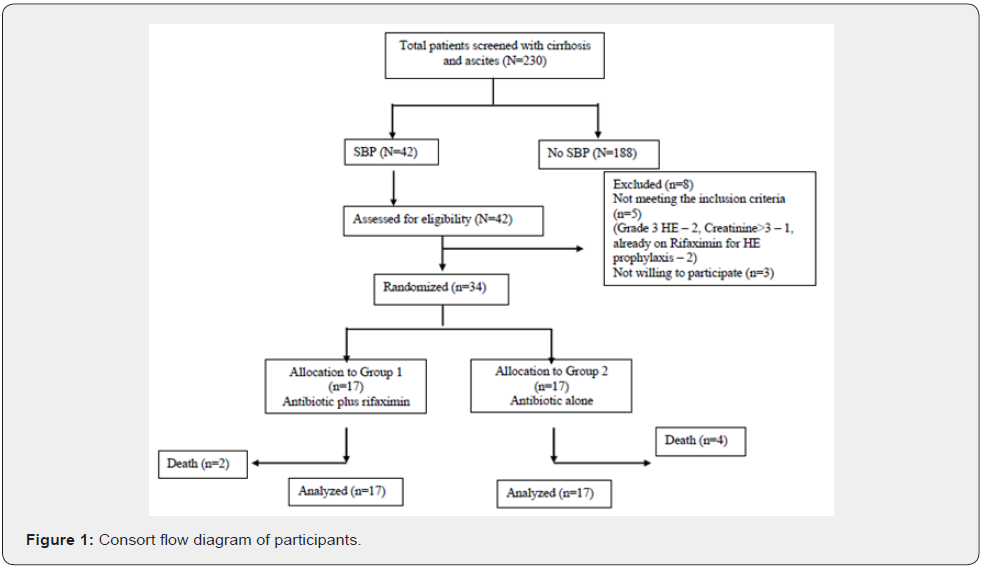
A total of 230 patients confirmed with a diagnosis of cirrhosis and ascites were assessed for SBP with diagnostic ascitic fluid analysis during the study period. Out of them, 42 had SBP and underwent assessment for eligibility. Eight patients were excluded from the study, among which five didn’t meet the inclusion criteria (2 of them had grade 3 HE, 1 of them had creatinine > 3mg/dl, and two were already on rifaximin for HE prophylaxis), and 3 were not willing to participate. Thiry-four patients were randomized into two groups, 17 in the antibiotic plus rifaximin group and 17 in the antibiotic alone group. During the study period, 2 of the patients died in the first group while 4 patients died in the second group. The total number of patients who were analyzed with the intention to treat analysis was 17 in each group (Figure 1).
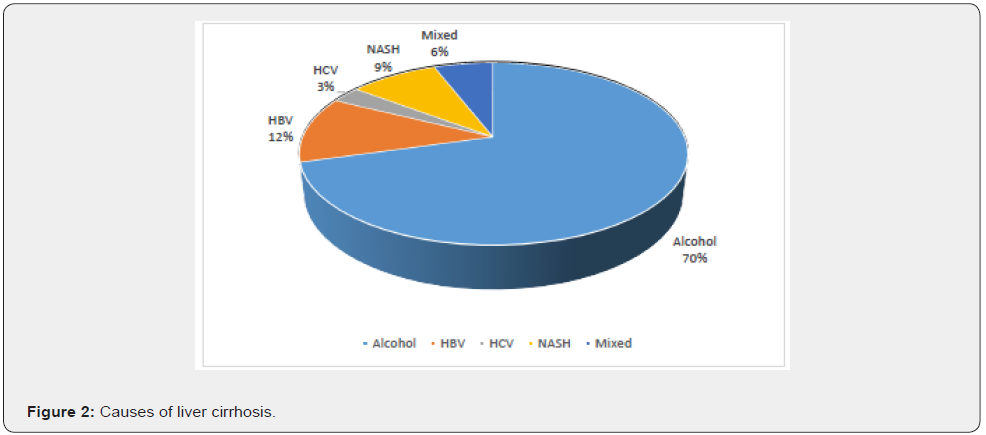
Out of a total of 34 patients, the mean age was 51.65(±10.21) years. Among them 8 (23%) were female while 26 (76%) were male. Etiologies of LC are shown in figure 2. The commonest etiology of liver cirrhosis was alcohol which was present in 70% of the cases.
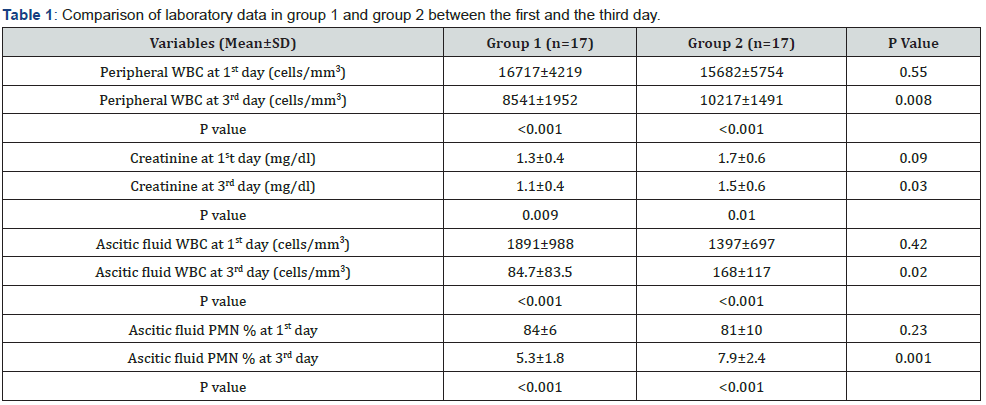
Peripheral WBC count was 16717±4219 and 8541±1952 cells/ mm3 on the first and third day, respectively, in group 1, whereas it was 15682±5754 and 10217±1491 cells/mm3 on the first and third day, respectively, in group 2. Both groups showed a significant reduction in peripheral WBC on the third day compared with the first day, with a P-value less than 0.001. Moreover, group 1 showed a marked decline in peripheral WBC on the third day compared with the decrease in group 2, with a P-value of 0.008. Similarly, serum creatinine was reduced significantly in both the groups on the third day compared with the first day (p=0.009 and p=0.01, respectively). Furthermore, group 1 had more reduction of creatinine on day 3 compared to group 2 (p=0.03).
Ascitic fluid WBC in group 1 was 1891±988 cells/mm3 on the first day and was 84.7±83.5 cells/mm3 on the third day of the therapy. Reduction in the ascitic fluid WBC was statistically significant with a p-value of less than 0.001. Group 2 also showed a notable drop in ascitic fluid WBC on the third day with a p-value of less than 0.001. Moreover, the reduction in ascitic fluid WBC count on the third day in group 1, compared with group 2 was statistically significant, with a p-value of 0.02. Similar changes were detected with ascitic fluid PMN%. Both groups showed a noteworthy reduction on the third day compared with the first day which was statistically significant in group 1 compared with group 2 on the third day (Table 1).
Outcome and complications
A total of 18 complications occurred at presentation or during the study period in the participants. Out of them, AKI was the most common complication occurring in 13 patients. HE was present in 3 patients at the initial presentation to the hospital, among which 2 of them had grade 1 HE while 1 of them had grade 2 HE. Nonfatal gastrointestinal bleeding occurred in two patients during the study period, which was managed as per the protocol. A total number of 6 patients died during the study period. Out of 6, 2 patients died in Group 1, whereas four died in Group 2. The reason for death in all the patients was sepsis with multi-organ failure.
There was no significant difference in outcome and complications between the two separate treatment groups (p=0.65 and p=0.35, respectively), although more patients died in Group 2. Duration of hospital stay was significantly longer in Group 2 compared with Group 1 (p=0.007). Though only 13 patients had AKI during the study period, the number of days to normalize the creatinine was shorter in Group 1 (3.2±0.9) than in Group 2 (4.5±1.5) but clinically not significant (p=0.10).
Discussion
The mean age of the study population was 51.65(±10.21) years. Eight (23%) of them were female, and 26 (76%) were male. This figure was similar to one study where the mean age was 55±2 years, and 65% were male [8]. The commonest etiology of liver cirrhosis was alcohol (70%). HBV was the cause in 12% of the cases, followed by NASH and HCV, present in 9% and 3%, respectively. A total of 2 (6%) of the patients had multiple etiologies. Similar to our previous studies, alcohol was the commonest cause of LC, followed by viral etiology. NASH is emerging as a cause of liver cirrhosis [17-19].
The most common symptom with which the patients presented as abdominal distension. It was present in 82% of the cases. Jaundice was present in 70% of the cases, followed by fever and abdominal pain, which were present in 64% and 41%, respectively. SBP should be suspected in patients with ascites due to advanced cirrhosis who develop symptoms such as fever, abdominal pain/tenderness, and altered mental status. Approximately 13 percent of patients with SBP have no signs or symptoms of infection at diagnosis [20].
In this study, a significant reduction in the peripheral and ascitic WBCs count after 48 hrs of antibiotic therapy in both treated groups was observed. Moreover, patients with cirrhosis in the rifaximin group showed a more significant decrease in WBC count. This marked reduction in WBC could refer to a more beneficial effect of combination therapy by adding rifaximin over antibiotics alone in the treatment of SBP. The positive outcome of adding rifaximin could be owing to the decline of BT and counteracting the enteric microorganism’s migration from the small intestine to the ascitic fluid. Assessment of reduction in peripheral and ascitic fluid WBC early in the course of treatment helps to find out if there is the presence of resistant organisms needing for change of antibiotics. Also, early reduction of WBC in both blood and ascitic fluid may prevent complications such as deterioration of renal function and development of hepatorenal syndrome, which is an important prognostic marker in patients with SBP.
A significant drop in serum creatinine was noted in both groups, after three days of antibiotic therapy. This reduction was more prominent among patients with cirrhosis in the rifaximin group than those who received antibiotics alone. In patients with cirrhosis with SBP, antibiotic treatment, and prevention of renal failure, are important prognostic factors for the reduction of mortality [21]. Significant improvement in renal function in both groups refers to the importance of early and proper initiation of antibiotic therapy in SBP to prevent deterioration of renal function and development of HRS. Adding rifaximin in combination therapy carries the advantage of being non-absorbable and lacking restriction for use, even in the presence of impaired renal function. Kalambokis et al. [22] reported improvement in systemic hemodynamics and renal function by intestinal decontamination with rifaximin for four weeks in patients with advanced cirrhosis. However, systemic hemodynamic effects of rifaximin after a few days need an evaluation and could not be equal to 4 weeks of therapy. Substitution of albumin, which plays an important role in the prevention of HRS, should be considered.
Six (18%) patients died during the study period. The reason for death in all the patients was sepsis with multi-organ failure. All the patients who died had renal failure as one of the components of MODS. The infection-related mortality from SBP is low with appropriate treatment [23]. Several reports found no infectionrelated deaths when treatment was started before a shock or frank renal failure. This study reinforces the recommendation to obtain ascitic fluid cultures immediately and then initiate empiric antimicrobial therapy in a patient with suspected SBP to maximize the patient’s chance of survival, specifically, if the patient has developed sepsis. Regardless of the short-term outcome related to the SBP, patients having severe liver disease have a poor long-term prognosis [18]. In-hospital, non-infection-related mortality may be as high as 20 to 40 percent, and one- and two-year mortality rates are approximately 70 and 80 percent, respectively [24]. In a large, nationwide database study of patients with cirrhosis, the three-year mortality rate for patients following hospitalization for SBP was 67 percent [25]. Thus, liver transplantation should be considered seriously for survivors of SBP who are otherwise good transplantation candidates.
This study favors the use of rifaximin in the treatment of SBP, on top of its use in preventing SBP. Considering the role of enteric flora in the pathophysiology of SBP has expanded the choice of rifaximin in the prevention and treatment of SBP and opened the door to the use of combination therapy in serious complications.
Conclusion
In our study, rifaximin plus Piperacillin-tazobactam showed more favorable effects, such as reducing peripheral WBC, creatinine, ascitic fluid WBC, and duration of hospital stay, when used for the treatment of SBP than Piperacillin-tazobactam only. However, the clinical effects and potential role of rifaximin and combination therapy in the management of SBP need to be clarified in further studies due to the smaller number of patients in this study.
References
- Garcia TG (2001) Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 120(3): 726-748.
- Nusrat S, Khan MS, Fazili J, Madhoun MF (2014) Cirrhosis and its complications: evidence- based treatment. World J Gastroenterol 20(18): 5442-5460.
- Groszmann RJ, Wongcharatrawee S (2004) The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 39(2): 280-282.
- Runyon BA (1994) Care of patients with ascites. N Engl J Med 330(5): 337-342.
- Obstein KL, Campbell MS, Reddy KR, Yang YX (2007) Association between model for end- stage liver disease and spontaneous bacterial peritonitis. Am J Gastroenterol 102(12): 2732-2736.
- Rimola A, García TG, Navasa M, Piddock LJ, Planas R, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol 32(1): 142-153.
- Angeli P, Guarda S, Fasolato S, Miola E, Craighero R, et al. (2006) Switch therapy with ciprofloxacin vs. intravenous ceftazidime in the treatment of spontaneous bacterial peritonitis in patients with cirrhosis: similar efficacy at lower cost. Aliment Pharmacol Ther 23(1): 75-84.
- Wiest R, Krag A, Gerbes A (2012) Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut 61(2): 297-310.
- Fernández J, Acevedo J, Castro M, Garcia O, De Lope CR, et al. (2012) Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 55(5): 1551-1561.
- Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, et al. (2007) Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 133(3): 818-824.
- Elfert A, Abo AL, Soliman S, Ibrahim S, Abd ES (2016) Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 28(12): 1450-1454.
- Koo HL, Du Pont HL (2010) Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol 26(1): 17-25.
- Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV (2012) Rifaximin for the prevention of spontaneous bacterial peritonitis. World J Gastroenterol 18(14): 1700-1702.
- Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, et al. (2013) Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol 28(3): 450-455.
- Mohammad AN, Elsamman MK, Zaghloul AM (2018) Rifaximin plus cefotaxime versus cefotaxime alone in treatment of spontaneous bacterial peritonitis in patients with cirrhosis. Egypt J Intern Med 30: 154-159.
- Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V (2015) Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 63(5): 1272-1284.
- Piano S, Brocca A, Mareso S, Angeli P (2018) Infections complicating cirrhosis. Liver Int 38(1): 126-133.
- Pradhan B, Hadengue A, Chappuis F, Chaudhary S, Baral D, et al. (2015) Alcoholic liver disease in Nepal: identifying homemade alcohol as a culprit. Clinical and experimental gastroenterology 8: 183-189.
- Mishra AK, Shrestha P, Bista NR, Bhurtel P, Bhattarai S, et al. (2009) Pattern of liver diseases. J Nepal Health Res Counc 7(1): 14-18.
- Tandon P, Garcia TG (2011) Renal dysfunction is the most important independent predictor of mortality in cirrhotic patients with spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol 9(3): 260-265.
- Hung TH, Tsai CC, Hsieh YH, Tsai CC (2015) The long-term mortality of spontaneous bacterial peritonitis in cirrhotic patients: A 3-year nationwide cohort study. Turk J Gastroenterol 26(2): 159-162.
- Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, et al. (2012) Rifaximin improves systemic hemodynamics and renal function in patients with alcohol- related cirrhosis and ascites. Clin Gastroenterol Hepatol 10(7): 815-818.
- Zapater P, Caño R, Llanos L, Ruiz AAJ, Pascual S, et al. (2009) Norfloxacin modulates the inflammatory response and directly affects neutrophils in patients with decompensated cirrhosis. Gastroenterology 137(5): 1669-1679.
- Runyon BA, Borzio M, Young S, Squier SU, Guarner C, et al. (1995) Effect of selective bowel decontamination with norfloxacin on spontaneous bacterial peritonitis, translocation, and survival in an animal model of cirrhosis. Hepatology 21(6): 1719-1724.
- Lee JM, Han KH, Ahn SH (2009) Ascites and spontaneous bacterial peritonitis: an Asian perspective. J Gastroenterol Hepatol 24(9): 1494-1503.






























