Retrospective Analysis of Exocrine Pancreatic Enzymes in Children with Eosinophilic Esophagitis and Malnutrition
Puanani Hopson1, Patricia Subnaik2, Karoly Horvath3,4 and Yamen Smadi4
1Mayo Clinic, Mayo Clinic Children’s Center Pediatric Gastroenterology and Hepatology Division, USA
2Golisano Children’s Hospital of Southwest Florida, USA
3The Florida State University, USA
4Center for Digestive Health and Nutrition, Arnold Palmer Hospital for Children, University of Florida at Orlando Health, USA
Submission:May 28, 2021; Published:June 14, 2021
*Corresponding author: Yamen Smadi, Center for Digestive Health and Nutrition, Arnold Palmer Hospital for Children, 60 W Gore Street, Orlando, FL 32806, USA
How to cite this article: Puanani H, Patricia S, Karoly H, Yamen S. Retrospective Analysis of Exocrine Pancreatic Enzymes in Children with Eosinophilic Esophagitis and Malnutrition. Adv Res Gastroentero Hepatol, 2021; 17(2): 555959. DOI: 10.19080/ARGH.2021.17.555959.
Abstract
Background: Malnourishment in eosinophilic esophagitis (EoE) often is multifactorial, due to feeding aversion, vomiting, poor diet, and dysphagia. Exocrine pancreatic insufficiency (EPI) also can lead to malnourishment in children. Our objective was to study EPI as a contributing cause of malnourishment in children with EoE.
Methods: We performed a retrospective review of records for pediatric patients (age 0-18 years) treated for EoE who underwent an endoscopic pancreatic function test (ePFT) due to malnutrition between July 2014 and June 2018 at the Eosinophilic Esophagitis Center of the Arnold Palmer Hospital for Children, Orlando, Florida.
Results: Thirty-six patients with EoE and malnutrition underwent ePFT and 16 were found to have EPI (10 males, average age 5.3 years). Five patients underwent follow-up ePFTs that showed EPI resolution after statistically significant weight gain (p = 0.035).
Conclusion: Poor growth in patients with EoE should prompt investigation into causes of failure to thrive, and exocrine pancreatic insufficiency should be considered as a contributing factor.
Keywords: Eosinophilic esophagitis; Exocrine pancreatic; Pediatric patients; Malnutrition
Abbreviations: EoE: Eosinophilic Esophagitis; FTT: Failure to Thrive; EPI: Exocrine Pancreatic Insufficiency; CF: Cystic Fibrosis; SDS: Shwachman-Diamond Syndrome; CP: Chronic Pancreatitis; SD: Standard Deviations
Introduction
Eosinophilic esophagitis (EoE) is a chronic, antigen-driven allergic disease characterized by eosinophil infiltration of the esophagus and consequent symptoms of esophageal dysfunction [1]. It was first reported in the late 1970s and is considered relatively rare. However, the incidence (3.7-12.8/100,000/year) and prevalence (22.7/100,000) of EoE have increased throughout the last 20 years [2,3].
Although dysphagia and food impaction are the most common EoE symptoms in adults, symptoms in children are non-specific and include food intolerance, failure to thrive (FTT), abdominal pain, nausea, and emesis [4,5]. Children with eosinophilic esophagitis often exhibit poor growth resulting from dysphagia, food aversion, restricted diet, and chronic vomiting [6]. Most children experience increased growth velocity once EoE is in remission; however, a subgroup continues to experience failure to thrive, despite effective control of EoE [6].
Exocrine pancreatic insufficiency (EPI) is the inability to digest nutrients due to a severe reduction in digestive enzymes secreted by the exocrine pancreas. The main clinical symptom of EPI is steatorrhea, due to an inability to digest fat [7,8]. However, since exocrine pancreatic enzymes digest all macronutrients (fat, protein, and carbohydrates)7 that play a key role in nutrient digestion, EPI also leads to malabsorption, malnutrition, and FTT in children [9], as well as to poor growth and mortality [10]. EPI exists in conditions, such as cystic fibrosis (CF), Shwachman- Diamond syndrome (SDS), and chronic pancreatitis (CP) [8,11,12] and several studies have reported that malnutrition can lead to EPI 13-15. The purpose of this investigation is to analyze exocrine pancreatic enzymes in a series of cases of children with EoE and malnutrition.
Methods
We performed a retrospective review of records for pediatric patients (age 0-18 years) treated for EoE who underwent an endoscopic pancreatic function test (ePFT) due to malnutrition between July 2014 and June 2018 at the Eosinophilic Esophagitis Center of the Arnold Palmer Hospital for Children. We collected clinical data, including age, z Scores (z) for weight, height, BMI, pancreatic enzyme profile, EoE treatment, and histopathology from esophageal biopsy. Diagnosis of EoE in our population is defined as symptoms consistent with esophageal dysfunction and esophageal epithelial eosinophilia >15 eosinophils per highpower field (eos/HPF) in esophageal biopsy while receiving highdose acid suppression as stated in the consensus guidelines for the period of the study 1. Malnourishment is defined as weight for height (W/H) ≤-2 standard deviations (SD).
Endoscopic pancreatic function test (ePFT).
In our center, we use ePFT to evaluate children for EPI as a cause of malnutrition, rather than indirect testing (such as fecal elastase) that has poor sensitivity for EPI, especially in children with selective enzyme deficiencies [16]. ePFT is a direct pancreatic function test performed during endoscopy that includes the collection of pancreatic fluid after pancreatic stimulation using secretin [16-18]. Synthetic human secretin (ChiRhoClin, Burtonsville, MD) is used as the hormonal stimulant and has been shown to be equivalent to biologic secretin [19-21]. After anesthesia induction, secretin (0.2mcg/kg) is given as an intravenous bolus over one minute. The endoscope is advanced to the duodenum and pancreatic fluid collection is obtained via an aspiration catheter inserted through the endoscopy channel and placed in close proximity to the ampulla of Vater. Four 1mL samples of pancreatic fluid are collected between 4-10 minutes after secretin administration. The collected pancreatic fluids are placed on dry ice and sent to the laboratory to be stored at -80⁰C until analysis. Analysis includes measurement of pH, protein, and enzyme activity of amylase, lipase, chymotrypsin, trypsin, and elastase. The peak pancreatic enzyme activity of the 4 collected specimens is used for reporting [16].
Enzyme assays
Our Pediatric Gastroenterology Translational Laboratory performed the pancreatic enzyme analysis. Given the limited data in the literature for standardized pancreatic enzyme values, we relied on the laboratory-defined reference ranges for pancreatic enzyme activity in our reference population via a rank and percentile calculation of more than 400 samples. Normal pancreatic enzyme activity for each enzyme individually was defined as activity above the third percentile.
The amylase activity (normal > 10.3 μmol/min/mL) was measured using Amylase LiquiColor reagent from STANBIO Laboratory (Boerne, TX, USA). The α-amylase in the sample hydrolyzes substrate, 4,6-ethylidene-G7p-nitrophenol (ETG7PNP), to generate 2-4 glucose (G) units with p-nitrophenol (PNP) fragments, then α-glucosidase (included with the reagent) hydrolyzes G2PNP and G3PNP to yield glucose and colored PNP product. The amount of the colored product is proportional to the activity of amylase. The amylase activity is calculated as the release of PNP (μmol) per minute, per milliliter of sample (μmol/min/ mL). Proteases were measured using chromogen-linked peptides as substrates: BAPNA (Benzoyl-DL-arginine-p-nitroaniline HCl) for trypsin (normal > 306.9 nmol/min/mL), Succinyl-Ala-Ala-Pro- Phe-p-nitroanilide for chymotrypsin (normal > 2.7 μmol/min/ mL) and Succinyl-Ala-Ala-Ala-p-nitroanilide for elastase (normal > 10.5 nmol/min/mL) (Sigma, St. Louis, MO). The reaction releases p-nitroanilide, a yellow color product, which was quantitatively measured at 405nm. Lipase activity (normal > 134.9 μmol/min/ mL) was determined by using Sekisui Lipase Color kit (Lexington, MA) and the reaction was monitored kinetically at 550nm as described in the protocol provided by the manufacturer.
Statistical analysis
Descriptive statistics was the primary analysis utilized. For those patients who had repeat ePFT we used t-test to compare change in weight, p value <0.05 was considered significant.
Ethical considerations
This retrospective study was performed in accordance with a protocol approved by the Arnold Palmer Medical Center Institutional Review Board.
Results
Thirty-six patients with EoE underwent ePFT due to malnutrition and 16 were found to have generalized or partial EPI. The study population included 10 males and 6 females, with average age at diagnosis of 5.3 years (range 1-17 years). Eleven patients (68%) were diagnosed with simultaneous EoE and EPI, four patients (25%) were diagnosed with EPI after EoE diagnosis, and 1 patient (6%) was diagnosed with EPI before EoE. Four patients had generalized pancreatic insufficiency and the rest had different pancreatic enzyme deficiencies (Table 1). For EoE management, 2 patients were treated with proton pump inhibitor (PPI) medications only, 5 patients were treated with elimination diet, and 9 patients were treated with topical swallowed corticosteroids (Table 1). Five patients underwent follow-up ePFT that demonstrated EPI resolution after statistically significant weight gain (p = 0.035). One patient underwent a repeat ePFT that demonstrated persistently low trypsin and chymotrypsin, despite significant improvement in growth parameters.
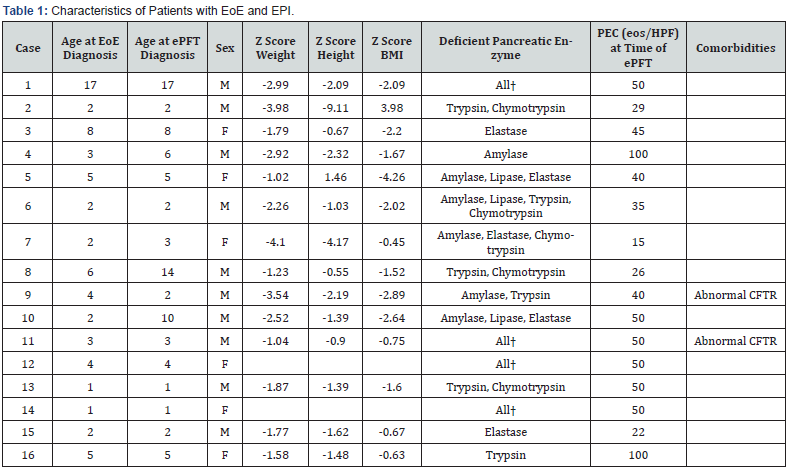
ePFT: Endoscopic Pancreatic Function Test; PEC: Peak Eosinophil Count; CFTR: Cystic fibrosis transmembrane conductance regulator †Amylase, Lipase, Elastase, Trypsin, and Chymotrypsin
Discussion
Our study represents the first published report of exocrine pancreatic enzyme analysis in patients with EoE and malnutrition. A study by Mukkada et al. 6 reported that 21% of children with EoE were diagnosed with FTT; however, there is no literature discussing whether children with FTT have EPI. In our retrospective observational review, we identified 16 patients with EoE and decreased exocrine pancreatic enzymes. All of these patients had clinical evidence of malnutrition. ePFT is performed during endoscopy for suspected EoE patients in our center if they also have malnutrition to investigate for EPI. We rely on ePFT because it is more sensitive than indirect exocrine pancreatic enzyme tests to investigate for EPI, particularly in a patient already undergoing EGD testing. One patient in our review had normal test results for fecal elastase, yet an abnormal ePFT. This is consistent with studies reporting fecal elastase testing as having poor diagnostic sensitivity for EPI, especially in children with selective enzyme deficiencies16.
Evidence that EPI can cause malnutrition is well documented. Yet, the relationship between malnutrition and the exocrine pancreas is still unclear [15], despite several previous and recent studies reporting that malnutrition can cause temporary EPI10- 14. It is important to note that in six patients, follow-up ePFT showed resolution of EPI after adequate weight gain. This is likely consequent to improved nutritional status. However, followup ePFT in one patient demonstrated persistently low trypsin and chymotrypsin, despite improved growth. Certainly, when a patient has EPI, one should consider the possibility of a syndromic etiology. None of the patients in this study had evidence of a syndromic EPI, such as cytopenia or bone abnormalities, that would suggest Schwachman-Diamond syndrome. Notably, the patients in this study who had other comorbidities (e.g., such as Cases 9 and 11 who had CFTR-related metabolic abnormality) had EPI resolution, once nutrition status improved.
Study limitations include retrospective data collection, small sample size, and lack of follow-up ePFT in some patients. Future prospective studies are needed to accurately explore the role of EPI in EoE. The etiology of EPI in patients with EoE is unclear. It is uncertain if one leads to the other or if they exist separately. There is evidence that malnutrition can cause EPI, and patients with EoE may become malnourished due to feeding difficulties. Whether there is any unknown connection between the two entities, other than malnutrition, remains unanswered. It is possible that eosinophils infiltrate the pancreas or that the cytokines associated with EoE cause some degree of enzyme derangement. Likewise, since the breakdown of proteins is compromised in EPI, this might introduce intact antigens to the immune system, leading to EoE. Overall, we conclude that poor growth and malnutrition in patients with EoE should prompt investigation into other causes of failure to thrive. Exocrine pancreatic insufficiency should be considered, since EoE and EPI can coexist.
References
- Liacouras CA, Furuta GT, Hirano I, Dan Atkins, Stephen E Attwood, et al. (2011) Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 128(1): 3-20.
- Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD (2014) Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol 12(4): 589-596.
- Mansoor E, Cooper GS (2016) The 2010-2015 Prevalence of Eosinophilic Esophagitis in the USA: A Population-Based Study. Dig Dis Sci 61(10): 2928-2934.
- Spergel JM, Brown-Whitehorn TF, Beausoleil JL, James Franciosi, Michele Shuker, et al. (2009) 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr 48(1): 30-36.
- Liacouras CA, Spergel J, Gober LM (2014) Eosinophilic esophagitis: clinical presentation in children. Gastroenterol Clin North Am 43(2): 219-229.
- Mukkada VA, Haas A, Maune NC, Kelley E Capocelli, Michelle Henry, et al. (2010) Feeding dysfunction in children with eosinophilic gastrointestinal diseases. Pediatrics 126(3): e672-677.
- Taylor CJ, Chen K, Horvath K, David Hughes, Mark E Lowe, et al. (2015) ESPGHAN and NASPGHAN Report on the Assessment of Exocrine Pancreatic Function and Pancreatitis in Children. J Pediatr Gastroenterol Nutr 61(1): 144-153.
- Lindkvist B (2013) Diagnosis and treatment of pancreatic exocrine insufficiency. World J Gastroenterol 19(42): 7258-7266.
- Bartels RH, van den Brink DA, Bandsma RH, Boele van Hensbroek M, Tabbers MM, et al. (2018) The Relation Between Malnutrition and the Exocrine Pancreas: A Systematic Review. J Pediatr Gastroenterol Nutr 66(2): 193-203.
- Littlewood JM, Wolfe SP, Conway SP (2006) Diagnosis and treatment of intestinal malabsorption in cystic fibrosis. Pediatr Pulmonol 41(1): 35-49.
- Shwachman H, Diamond LK, Oski FA, Khaw KT (1964) The Syndrome of Pancreatic Insufficiency and Bone Marrow Dysfunction. J Pediatr 65: 645-663.
- Nousia-Arvanitakis S (1999) Cystic fibrosis and the pancreas: recent scientific advances. J Clin Gastroenterol 29(2): 138-142.
- Brooks SE, Golden MH (1992) The exocrine pancreas in kwashiorkor and marasmus. Light and electron microscopy. West Indian Med J 41(2): 56-60.
- El-Hodhod MA, Nassar MF, Hetta OA, Gomaa SM (2005) Pancreatic size in protein energy malnutrition: a predictor of nutritional recovery. Eur J Clin Nutr 59(4): 467-473.
- Bartels RH, Meyer SL, Stehmann TA, Bourdon C, Bandsma RH, et al. (2016) Both Exocrine Pancreatic Insufficiency and Signs of Pancreatic Inflammation are Prevalent in Children with Complicated Severe Acute Malnutrition: An Observational Study. J Pediatr 174: 165-170.
- Wali PD, Loveridge-Lenza B, He Z, Horvath K (2012) Comparison of fecal elastase-1 and pancreatic function testing in children. J Pediatr Gastroenterol Nutr 54(2): 277-280.
- Abu-El-Haija M, Conwell DL (2018) Pancreatic Insufficiency: What Is the Gold Standard? Gastrointest Endosc Clin N Am 28(4): 521-528.
- Stevens T, Conwell DL, Zuccaro G, Frederick Van Lente, Rocio Lopez, et al. (2008) A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 67(3): 458-466.
- Somogyi L, Ross SO, Cintron M, Toskes PP (2003) Comparison of biologic porcine secretin, synthetic porcine secretin, and synthetic human secretin in pancreatic function testing. Pancreas 27(3): 230-234.
- Jowell PS, Robuck-Mangum G, Mergener K, Branch MS, Purich ED, et al. (2000) A double-blind, randomized, dose response study testing the pharmacological efficacy of synthetic porcine secretin. Aliment Pharmacol Ther 14(12): 1679-1684.
- Christ A, Werth B, Hildebrand P, Gyr K, Stalder GA, et al. (1988) Human secretin. Biologic effects and plasma kinetics in humans. Gastroenterology 94(2): 311-316.






























