Assessment of Renal Insufficiency among Non-Hypertensive and Non-Diabetic Adults with Non-Alcoholic Fatty Liver Disease
Elham Ahmed Hassan1*, Maiada Mohie El-dien Ibrahim1, Abeer Sharaf El-Din Abdel Rehim1, Mohamed-Eltaher A A Ibrahim1, Nahed A Makhlouf1, Hani Sayed Aboalam2, Asmaa Omar Ahmed3 and Samir Kamal Abdul-Hamid4
1Department of Gastroenterology and Tropical Medicine, Assiut University, Egypt
2Assiut Liver Center, Ministry of health, Egypt
3Department of Clinical pathology, Assiut University, Egypt
4Department of Internal Medicine and Nephrology, Assiut University, Egypt
Submission:May 25, 2021; Published:June 09, 2021
*Corresponding author:Elham Ahmed Hassan, MD, Professor, Department of Gastroenterology and Tropical Medicine, Al-Rajhi liver center, Assiut University Hospitals, Assiut 71515, Egypt
How to cite this article:Elham A H, Maiada M I, Abeer S A, Mohamed-Eltaher A A I, Nahed A M, et al. Assessment of Renal Insufficiency among Non- Hypertensive and Non-Diabetic Adults with Non-Alcoholic Fatty Liver Disease. Adv Res Gastroentero Hepatol, 2021; 17(2): 555958. DOI: 10.19080/ARGH.2021.17.555958.
Abstract
Background and Aim: Owing to their adverse outcomes, Association between non-alcoholic fatty liver disease (NAFLD) and renal dysfunction has a great appealing awareness. We aimed to assess renal changes and their association with liver fibrosis among non-hypertensive and non-diabetic patients with NAFLD.
Patients and Methods: This case-control study evaluated urine albumin-creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) using chronic kidney disease epidemiology collaboration (CKD-EPI) and modification of diet in renal disease (MDRD) in 100 NAFLD patients and 30 controls. Liver fibrosis was assessed using fibroscan and NAFLD Fibrosis Score (NFS). Renal insufficiency was defined as eGFR <90 mL/min/1.73m2.
Results: Total 41(41%) NAFLD patients had renal insufficiency where, 87.8% (36/41) of them had mild renal insufficiency (eGFR ≥60 and <90 mL/min/1.73m2). NAFLD had significantly more microalbuminuria and higher ACR and lower eGFR than controls and those with significant liver fibrosis exhibited the highest impairment. In partial correlation analysis, NFS associated with microalbuminuria and lower eGFR. Those with renal insufficiency were significantly associated with higher body mass index (BMI) and impaired lipid profile. Multivariate logistic regression analysis demonstrated independent association of NAFLD with significant fibrosis with renal insufficiency (OR=2.364, 95%CI=0.783-7.136) after adjusting for confounders (age, gender and BMI).
Conclusion: Renal changes are common in NAFLD. Severity of NAFLD is negatively associated with the kidney function. Obesity and dyslipidemia may explain this association. Monitoring of eGFR and albuminuria in those patients particularly with significant liver fibrosis would be beneficial for early recognition of mild renal insufficiency to prevent or delay adverse outcomes.
Keywords: Chronic kidney disease; Estimated glomerular filtration rate; Liver fibrosis; Non-alcoholic fatty liver disease; Renal insufficiency
Abbreviations: NAFLD: Non-Alcoholic Fatty Liver Disease; ACR: Albumin-Creatinine Ratio; eGFR: Estimated Glomerular Filtration Rate; MDRD: Modification of Diet in Renal Disease; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CVD: Cardiovascular Disease; CKD: Chronic Kidney Disease; CAP: controlled Attenuation Parameter; BMI: Body Mass Index; DM: Diabetes Mellitus; TG: Triglycerides; HDL: High Density Lipoprotein; ACR: Albumin to Creatinine Ratio; pSWE: Point Shear Wave Elastography; TE: Transient Elastography; SD: STANDARD DEVIATION; RAAS: Renin-Angiotensin-Aldosterone System
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a rising public health concern worldwide, where it is now believed to be a hepatic manifestation of metabolic syndrome besides the concomitance of significant co-morbidities as hypertension, obesity, diabetes, and atherogenic dyslipidemia [1]. However, there is a greater risk and increasing proof of its association with serious extrahepatic complications as cardiovascular disease (CVD) and chronic kidney disease (CKD) and hence liver-related morbidity and mortality [2].
CKD is considered a major threat of end-stage renal disease, with prolonged reduction in the glomerular filtration rate (GFR), structural or functional renal anomalies and results in high morbidity, mortality [3]. Proper identification and management of early stages with mild renal insufficiency could prevent or delay the adverse outcomes [4].
There is a great appealing awareness of an impending association between NAFLD and CKD. There is an evidence of persistence even after liver transplantation, where 35% of NAFLDrelated cirrhosis progressed to stage 3-4 CKD within two years after transplantation compared to 10% of patients transplanted for other etiologies [5].
Several studies displayed the association between NAFLD and CKD [6-9] however, to our knowledge, studies regarding renal changes especially early stages with mild renal insufficiency in NAFLD patients are lacking especially in our region. Therefore, this work aimed to assess these renal changes and their association with liver fibrosis among non-hypertensive and non-diabetic patients with NAFLD.
Patients and Methods
Study design
This case-control study was carried out at Assiut University Hospitals, Egypt from January 2019 to January 2020. The Local Ethics Committee of Faculty of Medicine, Assiut University, Egypt approved the study (IRB No 17100479) in accordance with the previsions of the Declaration of Helsinki. The study was registered on clinical trials. gov with ID. NCT03487068. Written informed consent was obtained from all participants.
Study population
This study consecutively included 100 adult patients with NAFLD recruited from outpatient clinics and inpatient wards of the Gastroenterology and Tropical Medicine Department, Al- Rajhi Liver Center, Assiut University Hospitals, and Assiut Liver Center, ministry of Health, Assiut, Egypt from January 2019 to January 2020. Diagnosis of NAFLD was based on i) clinical findings with special stress on manifestations of chronic liver disease e.g., malaise, right upper quadrant abdominal pain, and/ or hepatomegaly, ii) biochemical findings including chronically elevated serum liver enzymes, iii) ultrasonographic hepatic steatosis findings including liver hyperechogenicity relative to kidneys, poor visualization of intrahepatic vessel borders and diaphragm and ultrasound beam attenuation [10]. iv) controlled attenuation parameter (CAP) determination using transient elastography (TE; Fibroscan®, Echosens, Paris, France) where, NAFLD was defined by CAP values ≥238 dB/m [11].
Thirty controls had normal ultrasonographic liver and kidney findings and were sex, age and body mass index (BMI) matched with patients. They were selected randomly from relatives of the patients. Patients with evidence of diabetes mellitus (DM), hypertension, advanced kidney disease, cardiovascular events, cirrhosis, liver disease rather than NAFLD (e.g., viral hepatitis, hepatocellular carcinoma, autoimmune hepatitis, Wilson’s disease and hemochromatosis), receiving any nephrotoxic drugs or alcohol consumption (>20 g/day) were excluded.
Methods
At study entry, a thorough medical history and physical examination were taken for information collection; for example age, gender, smoking, comorbidities like diabetes, medication use, abdominal pain, BMI, waist circumference and signs of chronic liver disease were noted. Venous blood samples were collected in the morning after an overnight fast for laboratory investigations including complete blood picture, liver function (serum bilirubin, albumin, enzymes and INR), fasting blood glucose, lipid profile [serum cholesterol, triglycerides (TG), high density lipoprotein (HDL)], and kidney function (serum creatinine and blood urea). All laboratory tests were carried out in Core LAb Unit, Clinical Pathology, Assiut University Hospital, Egypt by using full automated system of ADVIA 2120i (Hematology system) according to their manufacturer’s instruction. In addition, early morning urine samples were collected for measuring urinary micro-albumin and urine albumin to creatinine ratio (ACR).
All patients underwent point shear wave elastography (pSWE) using the Philips iU 22 Ultrasonic Diagnostic Apparatus (Bothell, WA; ElastPQ) with a diagnostic probe frequency of 3.5~5.0 MHz and Transient elastography (TE) (Fibroscan®) for assessment of liver fibrosis.
By data collection, the estimated glomerular filtration rate (eGFR) and hepatic fibrosis scores were calculated as the following
a) eGFR by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)= 141 x min(SCr/κ, 1)α x max(SCr /κ, 1)- 1.209 x 0.993Age x 1.018 [if female] x 1.159 [if Black]. eGFR (estimated glomerular filtration rate) = mL/min/1.73 m2, SCr (standardized serum creatinine) = mg/dl, κ= 0.7 (females) or 0.9 (males), α= -0.329 (females) or -0.411 (males), min= indicates the minimum of SCr/κ or 1, max= indicates the maximum of SCr/κ or 1, age= years [12].
b) eGFR by Modification of Diet in Renal Disease (MDRD)= 175 x (SCr)-1.154 x (age)-0.203 x 0.742 [if female] x 1.212 [if Black]. eGFR= mL/min/1.73 m2, Scr= mg/dl, age= years [13].
c) According to the categorization of the National Kidney Foundation Practice Guidelines [13], normal kidney function, and renal insufficiency were defined as eGFR ≥ 90, and < 90 ml/ml/ min/1.73m2 respectively where, mild, moderate and severe renal insufficiency were defined as eGFR ≥60 and <90, and ≥30 and < 60, and < 30 ml/min/1.73m2 respectively.
d) Urine albumin-creatinine ratio (ACR): normal value was approximately 10 mg/g, < 30 mg/g was mildly increased; 30-300 mg/g was moderately increased and > 300 mg/g was severely increased [14].
e) NAFLD Fibrosis Score (NFS)= -1.675 + 0.037 × age (year) + 0.094 × BMI (kg/m2) + 1.13 × IFG/diabetes (yes= 1, no= 0) + 0.99 × AST/ALT ratio - 0.013 × platelet count (×109/L) - 0.66 × albumin (g/dL). NFS < -1.5 for low, NFS ≥ -1.5 to NFS < 0.67 for intermediate and NFS ≥ 0.67 for high probability of fibrosis at baseline [15].
Statistical analysis
Statistical analyses were conducted using SPSS for windows version 16 (IBM Corp., Armonk, NY, USA) and Microsoft Excel 2010. The Shapiro-Wilk test of Normality was used to test the normality of data. Continuous data was expressed as means ± standard deviation (SD) or median and range and was compared using Student’s t test or Mann-Whitney U test. Categorical variables were expressed as a percentage and compared using chi-squared (χ2) or Fisher’s exact probability test. Spearman partial correlation was used to find correlation between renal and NAFLD-related parameters after adjusting for age and sex. Multivariate logistic regression analysis to adjust for confounding variables was conducted to estimate the odds ratio (OR) and 95% confidence intervals (95% CIs) of renal changes including mild renal insufficiency according to the presence of NAFLD. All tests were two-tailed and statistical significance was assessed at < 0.05.
Results
Characteristics of the study population
This study compromised 100 NAFLD patients; 55 females and 45 males with a mean age of 42.8 ± 10.9 years. The control group was formed of 14 females and 16 males with a mean age of 40 ± 13.4 (19 – 65) years. Based on liver stiffness measurement, mild, moderate and severe liver fibrosis were found in 55 (55%), 41 (41%) and four (4%) patients, respectively. For the purpose of analysis, cases of moderate liver fibrosis and those with severe fibrosis were grouped together in a group of significant fibrosis (n= 45). Demographic and laboratory characteristics of the study population were summarized in Table 1.
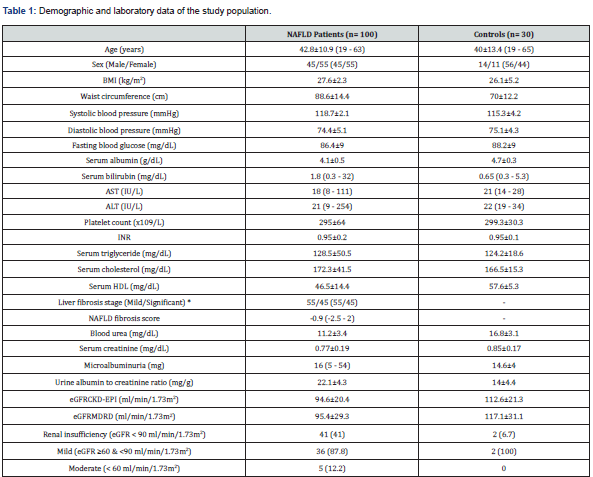
Data are expressed as mean±standard deviation (range) for parametric data, median (range) for non-parametric data, or n (%) for qualitative data.
*Significant liver fibrosis included moderate (n= 41) and severe (n= 4) liver fibrosis stages
AST: Aspartate Transaminase; ALT: Alanine Transaminase; BMI: Body Mass Index; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; eGFR: Estimated Glomerular Filtration Rate; HDL: High Density Lipoprotein; INR: International Normalized Ratio; NAFLD: Non-Alcoholic Fatty Liver Disease; MDRD: Modification of Diet in Renal Disease Study
Comparison of renal changes between subjects with and without NAFLD
In this study, NAFLD group had significantly more microalbuminuria and higher mean ACR (P < 0.001 for both) and significantly lower mean eGFR level measured by CKD-EPI and MDRD equations (P = 0.001 and 0.004 respectively) compared with normal liver subjects, and those with significant liver fibrosis exhibited the highest impairment, as shown in Table 2. In addition, two NAFLD cases had moderate increased albuminuria (ACR between 30 - 300 mg/g) where; their ACR levels were 32 and 33 mg/g respectively.
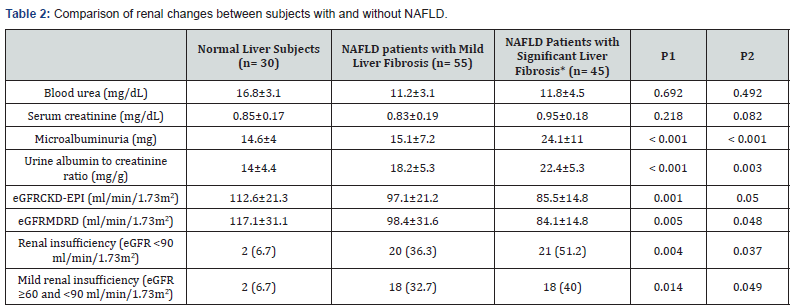
Data are expressed as mean±standard deviation (range) for parametric data, median (range) for non-parametric data, or n (%) for qualitative data.
P < 0.05 = significant.
*Significant liver fibrosis included moderate (n= 41) and severe (n=4) liver fibrosis stages
P1: P- value for differences between three groups
P2: P-value for differences between NAFLD patients with significant liver fibrosis and those with mild liver fibrosis
CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; eGFR: Estimated Glomerular Filtration Rate; MDRD: Modification of Diet in Renal Disease Study Equation; NAFLD: Nonalcoholic Fatty Liver Disease
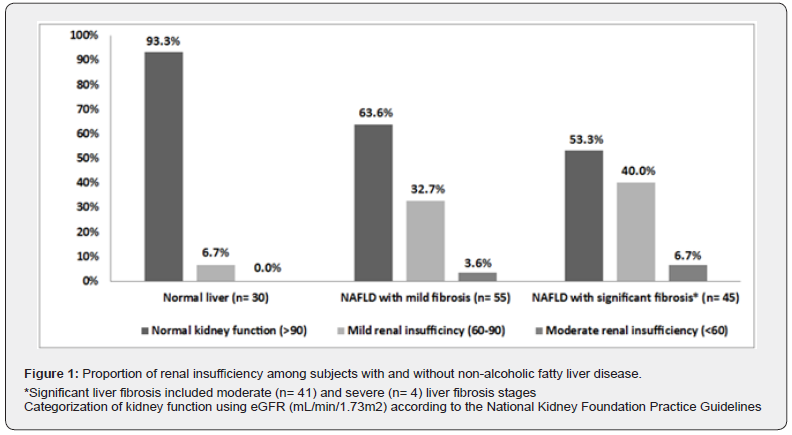
Out of 100 NAFLD patients, 41 (41%) patients had renal insufficiency (eGFR < 90 mL/min/1.73 m2). Moreover, according to the categorization of the National Kidney Foundation Practice Guidelines [13], the majority of NAFLD cases (59/100, 59%) had normal kidney function (eGFR ≥90 ml/min/1.73m2), 36 (36%) and five (5%) NAFLD cases had mild and moderate renal insufficiency respectively. However, no cases had severe renal insufficiency.
We found that subjects with NAFLD had a higher proportion of renal insufficiency compared to those without (41% vs. 6.7%, P = 0.004) with the highest proportion was observed among patients with significant fibrosis. Besides, the proportion of mild renal insufficiency rose with the increase of liver fibrosis staging (P = 0.014) as shown in Table 2. The proportion of renal insufficiency among subjects with and without NAFLD was shown in Figure1.
Partial correlations between renal and NAFLD-related parameters
Correlations between renal and NAFLD-related parameters after adjusting for age and sex among NAFLD patients revealed significant correlations between serum creatinine and BMI (r = 0.306; P = 0.025) and microalbuminuria and NFS (r = 0.405; P = 0.005) and HDL (r = 0.343; P = 0.018) and negative correlation between microalbuminuria and AST (r = -0.301; P = 0.042) and platelets (r = -0.423; P = 0.003). In addition, eGFRCKD-EPI and eGFRMDRD were negatively correlated with BMI (r = -0.337, P = 0.009 and r = -0.421, P = 0.003 respectively) and serum cholesterol (r = -295, P = 0.046 and r = -0.288, P = 0.05 respectively). In addition, eGFRCKD-EPI was negatively correlated with NFS (r = -0.315; P = 0.031).
Comparison between NAFLD regarding the presence of renal insufficiency
In this study, NAFLD patients with renal insufficiency had higher BMI (P = 0.013) and serum cholesterol levels (P = 0.028) than those with normal kidney function. Also, there was a tendency towards impaired levels of other lipid parameters in patients with renal insufficiency but without statistical significance as shown in Table 3. Furthermore, renal insufficiency was observed in about half of NAFLD cases with significant fibrosis (51.2%).
Multivariable logistic regression analysis for renal insufficiency as a dependent variable regarding the presence of NAFLD
By using adjusted model of multivariate logistic regression analysis for age, gender and BMI, subjects with NAFLD was significantly associated with the increased risk of renal insufficiency and mild renal insufficiency compared to those without NAFLD (OR= 1.357, 95% CI= 0.521-3.533 and OR= 1.913, 95% CI= 0.734-4.987; P < 0.001 respectively). Furthermore, renal insufficiency and mild renal insufficiency were positively associated with enhancing fibrosis of NAFLD (OR= 2.364, 95% CI= 0.783-7.136; P = 0.002 and OR= 2.095, 95% CI= 0.967-2.508; P = 0.001) for NAFLD patients with significant fibrosis vs. normal liver subjects (Table 4).
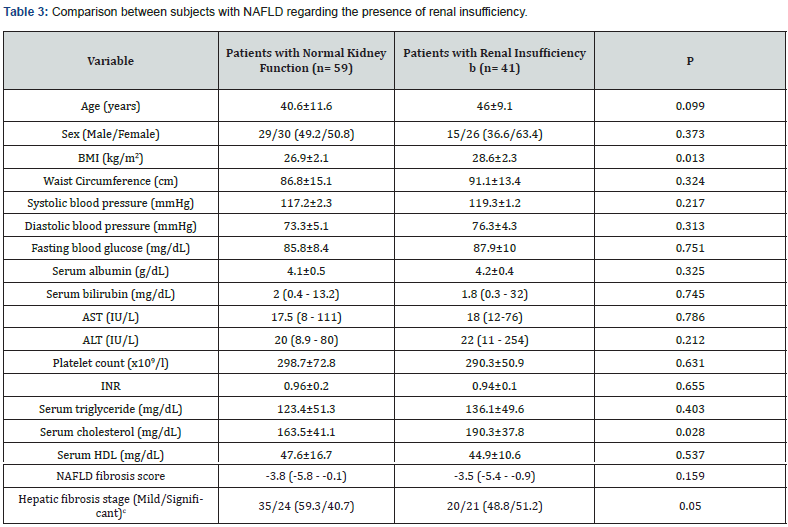
Data are expressed as mean±standard deviation (range) for parametric data, median (range) for non-parametric data, or n (%) for qualitative data.
P < 0.05 = significant.
aNormal kidney function= eGFR ≥ 90 ml/min/1.73m2
bRenal insufficiency = eGFR < 90 ml/min/1.73m2
cSignificant liver fibrosis included moderate (n= 41) and severe (n= 4) liver fibrosis stages
ALP: Alkaline Phosphatase; AST: Aspartate Transaminase; ALT: Alanine Transaminase; BMI: Body Mass Index; HDL: High-Density Lipoprotein; INR, International Normalized Ratio; NAFLD: Nonalcoholic Fatty Liver Disease
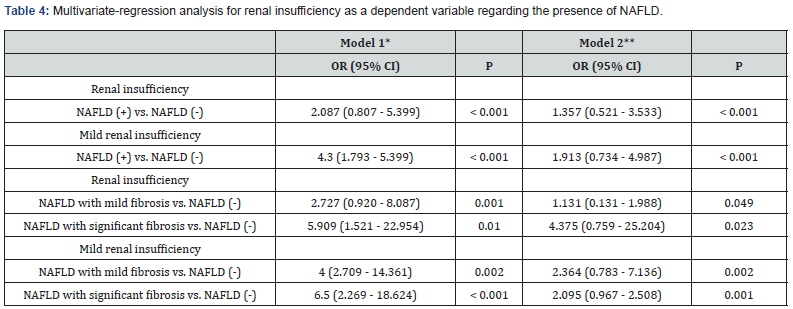
*Model 1 was unadjusted. **Model 2 was adjusted for age, sex, BMI. P < 0.05 = significant.
CI: Confidence Interval; NAFLD: Nonalcoholic Fatty Liver Disease; OR: Odds Ratio; vs: Versus
Discussion
This study aimed to highlight the renal dysfunction in nondiabetic, non-hypertensive NAFLD patients, where, both share some common comorbidities [1]. Similar to previous studies [8,16,17], the present study demonstrated that NAFLD patients had significant increased risk of renal insufficiency even after adjusting the potential confounders as age, gender and BMI (OR= 1.357, 95% CI = 0.521 - 3.533, p < 0.001).
This work identified that 41% of NAFLD patients had renal insufficiency which was within the range of the previous estimates (20-55%) [6,7,18,19]. In addition, the majority of them (87.8%) had mild renal insufficiency (eGFR; ≥60 and <90 ml/min/1.73m2) that was supported by the previous studies that may afford further evidence of the associations between NAFLD and the early stage of renal insufficiency [8,20].
In this study, the observed significant lower eGFR and higher albuminuria among NAFLD patients goes with what has been reported previously where the highest impairment seen with increasing severity of NAFLD (mainly liver fibrosis) supporting a role for NAFLD in the pathogenesis and/or progression of CKD [21-23]. Qin et al. [24] reported that a higher value of liver stiffness was a potential risk of CKD. In addition, eGFR levels in NAFLD patients were negatively correlated with the NFS; the higher the score, the lower the eGFR levels. Additionally, microalbuminuria was significantly related with NFS and negatively related with AST and platelets. These results were compatible with previous studies that reported a close correlation between renal dysfunction and the amount of liver damage caused by fatty infiltration [21,24,25].
Former studies demonstrated that the stage of fibrosis in NAFLD patients determines the risk of extrahepatic diseases including CVD and CKD, a positive association between NFS and CKD, and a positive correlation between microalbuminuria as a marker of kidney damage and liver fibrosis in non-diabetic patients with NAFLD [21,26,27]. In contrast, Akahane et al. [28] showed that NAFLD itself is not an independent risk factor for CKD but comorbidities as obesity, hypertension and hyperuricemia were independently associated with CKD.
Similar to previous studies [29-31], we found that renal insufficiency in NAFLD patients had significantly higher BMI and impaired lipid profile than those with normal renal function. Akahane et al. [28] revealed that the prevalence of CKD in NAFLD patients was significantly high in obese subjects. Moreover, Mahaling et al. [32] stated that in NAFLD, serum total cholesterol, TG, and LDL cholesterol showed a stepwise increase from simple steatosis to NASH, while lowest levels of HDL and cholesterol were seen in NASH patients.
Steatotic and inflamed liver may play a direct role in the development or progression of CKD through release of pathogenic mediators, exacerbation of insulin resistance, VLDL lipoprotein over-secretion and induction of atherogenic dyslipidemia that promote glomerular injury and mesangial cell proliferation [17,23]. In addition, NAFLD and CKD seem to share common metabolic risk factors and pathologic processes. Different obesityassociated mechanisms including lipotoxicity, oxidative stress, enhanced pro-inflammatory cytokine and renin-angiotensinaldosterone system (RAAS) axis activation are associated with the development of proteinuria and pathologic renal findings even mild renal or subclinical insufficiency in the absence of diabetes and hypertension suggesting that NAFLD may promote CKD independently of coexisting risk factors [17]. Recently, Byrne and Targher documented that NAFLD may promote kidney injury mostly through accelerated atherothrombosis by releasing inflammatory and procoagulant factors from steatotic liver [16].
Some limitations were addressed in this study. It was small sample-sized, hospital-based study so the results may not represent the general population. Despite different non-invasive modalities were used to diagnose NAFLD (ultrasonography and fibroscan-CAP), they may not be accurate for measuring mild hepatic steatosis. On the other hand, liver biopsy, which is the gold standard for the diagnosis of NAFLD, is not feasible. Similar to previous studies, eGFR was used to define renal dysfunction rather than more precise measures of kidney function like directly measured GFR and renal biopsy. Large-scale cohort samples are needed to confirm these findings, to identify the pathogenic pathways of CKD in patients with NAFLD and to study the effect of therapeutic interventions and their outcome.
Conclusion
Our findings suggest that renal dysfunction is highly prevalent in NAFLD patients. The severity of NAFLD is negatively correlated with the kidney function. Assessment of eGFR and albuminuria seems to be currently the most relevant methods for this purpose. Common metabolic risk factors for both diseases e.g., obesity and dyslipidemia can explain this association. Screening NAFLD patients particularly with significant liver fibrosis is recommended for early recognition and proper management of impaired kidney function to prevent disease progression.
References
- Lonardo A, Sookoian S, Chonchol M, Loria P, Targher G (2013) Cardiovascular and systemic risk in nonalcoholic fatty liver disease-atherosclerosis as a major player in the natural course of NAFLD. Curr Pharm Des 19(29): 5177-5192.
- Anstee QM, Targher G, Day CP (2013) Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 10(6): 330-344.
- James MT, Hemmelgarn BR, Tonelli M (2010) Early recognition and prevention of chronic kidney disease. Lancet 375(9722): 1296-1309.
- Oh SW, Baek SH, Kim YC, Goo HS, Heo NJ, et al. (2012) Mild decrease in estimated glomerular filtration rate and proteinuria are associated with all-cause and cardiovascular mortality in the general population. Nephrol Dial Transplant 27(6): 2284-2290.
- Singal AK, Salameh H, Kuo YF, Wiesner RH (2014) Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation 98(2): 216-221.
- Sirota JC, McFann K, Targher G, Chonchol M, Jalal DI (2012) Association between nonalcoholic liver disease and chronic kidney disease: an ultrasound analysis from NHANES 1988–1994. Am J Nephrol 36(5): 466-471.
- Xia MF, Yan HM, He WY, Li XM, Li CL, et al. (2012) Standardized ultrasound hepatic/renal ratio and hepatic attenuation rate to quantify liver fat content: an improvement method. Obesity (Silver Spring) 20(2): 444-452.
- Nam GE, Hwang SY, Chung HS, Choi JH, Lee HJ, et al. (2018) Implication of Nonalcoholic Fatty Liver Disease, Metabolic Syndrome, and Subclinical Inflammation on Mild Renal Insufficiency. Int J Endocrinol 2018: 1835486.
- Chen PC, Kao WY, Cheng YL, Wang YJ, Hou MC, et al. (2020) The correlation between fatty liver disease and chronic kidney disease. J Formos Med Assoc 119(1 Pt 1): 42-50.
- Mehta SR, Thomas EL, Bell JD, Johnston DG, Taylor-Robinson SD (2008) Non-invasive means of measuring hepatic fat content. World J Gastroenterol 14(22): 3476-3483.
- Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, et al. (2010) Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 36(11): 1825-1835.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9): 604-612.
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, et al. (2003) National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, classification, and stratification. Ann Intern Med 139(2): 137-147.
- American Diabetes Association (2007) Standards of medical care in diabetes-2007. Diabetes Care 30(Suppl 1): S4-S41.
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, et al. (2007) The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45(4): 846-854.
- Byrne CD, Targher G (2020) NAFLD as a driver of chronic kidney disease. J Hepatol 72(4):785-801.
- Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, et al. (2014) Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 11(7): e1001680.
- Targher G, Byrne CD (2017) Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol 13(5): 297-310.
- Chang Y, Ryu S, Sung E, Woo HY, Oh E, et al. (2008) Nonalcoholic fatty liver disease predicts chronic kidney disease in non-hypertensive and nondiabetic Korean men. Metabolism 57(4): 569-576.
- Li G, Shi W, Hug H, Chen Y, Liu L, et al. (2012) Nonalcoholic fatty liver disease associated with impairment of kidney function in nondiabetes population. Biochem Med (Zagreb) 22(1): 92-99.
- Yilmaz Y, Alahdab YO, Yonal O, Kurt R, Kedrah AE, et al. (2010) Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: association with liver fibrosis. Metabolism 59(9):1327-1330.
- Onnerhag K, Hartman H, Nilsson PM, Lindgren S (2019) Non-invasive fibrosis scoring systems can predict future metabolic complications and overall mortality in non-alcoholic fatty liver disease (NAFLD). Scand J Gastroenterol 54(3): 328-334.
- Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, et al. (2010) Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol 5(12): 2166-2171.
- Qin S, Wang S, Wang X, Wang J (2019) Liver stiffness assessed by transient elastography as a potential indicator of chronic kidney disease in patients with nonalcoholic fatty liver disease. J Clinl Lab Anal 33(2): e22657.
- Liu H, Liu J, Kuo K (2019) Association of nonalcoholic fatty liver and chronic kidney disease: An analysis of 37,825 cases from health checkup center in Taiwan. Ci Ji Yi Xue Za Zhi 32(1): 65-69.
- Adams LA, Anstee QM, Tilg H, Targher G (2017) Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 66(6): 1138-1153.
- Kiapidou S, Liava C, Kalogirou M, Akriviadis E, Sinakos E (2019) Chronic kidney disease in patients with non-alcoholic fatty liver disease: What the Hepatologist should know? Ann Hepatol 19(2): 134-144.
- Akahane T, Akahane M, Namisaki T, Kaji K, Moriya K, et al. (2020) Association between Non-Alcoholic Fatty Liver Disease and Chronic Kidney Disease: A Cross-Sectional Study. J Clin Med 9(6): 1635.
- Fierbinteanu-Braticevici C, Baicus C, Tribus L, Papacocea R (2011) Predictive factors for nonalcoholic steatohepatitis (NASH) in patients with nonalcoholic fatty liver disease (NAFLD). J Gastrointestin Liver Dis 20(2): 153-159.
- Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, et al. (2009) The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 50(6): 1827-1838.
- Miura K, Ohnishi H (2012) Nonalcoholic fatty liver disease: from lipid profile to treatment. Clin J Gastroenterol 5(5): 313-321.
- Mahaling DU, Basavaraj MM, Bika AJ (2013) Comparison of lipid profile in different grades of non-alcoholic fatty liver disease diagnosed on ultra sound. Asian Pac J Trop Biomed 3(11): 907-912.






























