MRI Guided Diagnosis of Acute Cellular Rejection in a Case of ABO Incompatible Liver Transplant; A Case Report and Review of its Application in Living Donor Liver Transplant
Sapana Verma1*, Subhash Gupta1*, Ruchi Rastogi2, Anjali Kakati Gupta3, Rajesh Dey1, Sharat Varma1, Dibyajyoti Das1 and Shaleen Agarwal1
1Centre for Liver and Biliary Sciences, Max Super Speciality Hospital, India
2Department of Radiology, Max Super Speciality Hospital, India
3Department of Pathology, Max Super Speciality Hospital, India
*Both authors have equally contributed to this paper
Submission:April 09, 2021; Published:June 01, 2021
*Corresponding author:Dr. Sapana Verma, Centre for Liver and Biliary Sciences, Max Super Speciality Hospital, Saket 1, Press Enclave Road, Saket, New Delhi- 110 017, India
How to cite this article:Sapana V, Subhash G, Ruchi R, Anjali Kakati G, Rajesh D, et al. MRI Guided Diagnosis of Acute Cellular Rejection in a Case of ABO Incompatible Liver Transplant; A Case Report and Review of its Application in Living Donor Liver Transplant. Adv Res Gastroentero Hepatol, 2021; 17(2): 555956. DOI: 10.19080/ARGH.2021.17.555956.
Abstract
The diagnosis of acute cellular rejection (ACR) is generally not difficult in ABO compatible Living donor liver transplant (LDLT). However, in paediatric cases and in ABO incompatible liver transplant, if the presentation is atypical with fever and minimal derangements in liver function tests or if it is fulminant, the diagnosis may not either be suspected or be made sufficiently in time for quick treatment. Liver biopsy is the gold standard but is invasive, cannot be repeated frequently and results may take 24 to 48 hours even in the most efficient setups. Magnetic resonance imaging/ Magnetic resonance cholangiopancreatography (MRI/MRCP) examination is used frequently for diagnosing biliary complications but there are hardly any reports in liver transplant for the use of this modality for diagnosis of rejection. Here we report a case of an ABO incompatible liver transplant in a paediatric recipient where MRI/MRCP examination was carried out to evaluate the biliary tree which gave us the clue for severe rejection and treatment could be started even before the biopsy report was available. We suggest that MRI/MRCP scan should be included in routine postoperative evaluation of graft dysfunction in LDLT.
Keywords: Acute cellular rejection; ABO incompatible transplant; MRI/MRCP; Paediatric liver transplant
Abbreviations: ACR: Acute Cellular Rejection; LFT: Liver Function Test, LDLT: Living Donor Liver Transplantation, MRI: Magnetic Resonance Imaging; MRCP: Magnetic Resonance Cholangiopancreatography
Introduction
The postoperative course of ABO incompatible liver transplant is usually stormy and requires close monitoring for rejection till accommodation occurs. These patients are also prone to technical issues such as hepatic artery thrombosis, biliary complications and infected fluid collections [1]. A prompt diagnosis is the key to successful treatment. The diagnosis of rejection cannot be made without a liver biopsy. Occasionally, liver biopsy may not be feasible if the platelet counts are low or if there is derangement of clotting factors [2]. Radiological imaging such as duplex ultrasound will rule out technical issues such as vascular thrombosis. A MRI/MPCP examination will rule out biliary complications [3]. However, MR imaging has not been studied for the diagnosis of rejection. We therefore report here a case where the MRI/MRCP examination helped us in making the diagnosis of acute cellular rejection and treatment could be started even before the biopsy results were out.
Case Report
A 12- year old girl with a diagnosis of Caroli’s Disease from the age of 5 years presented to us with high fever, ascites, biliary obstruction and liver failure. She had multiple previous admissions for cholangitis and as the bile ducts were not suitable for biliary bypass procedures, she was evaluated for living donor liver transplantation. Unfortunately, the only suitable donor was her elder sister with a different blood group. A B to O incompatible living donor liver transplant was planned following Rituximab desensitization therapy. The donor, although asymptomatic tested positive for coronavirus disease (COVID 19) polymerase chain reaction (PCR) swab and therefore the operation was postponed. The recipient remained in and out of hospital for the next 6 weeks for multiple episodes of fever and ascites. Finally, once the COVID tests were negative twice and a total of 28 days were over, the recipient underwent sessions of plasmapheresis to bring the anti B titres to less than 1:32.
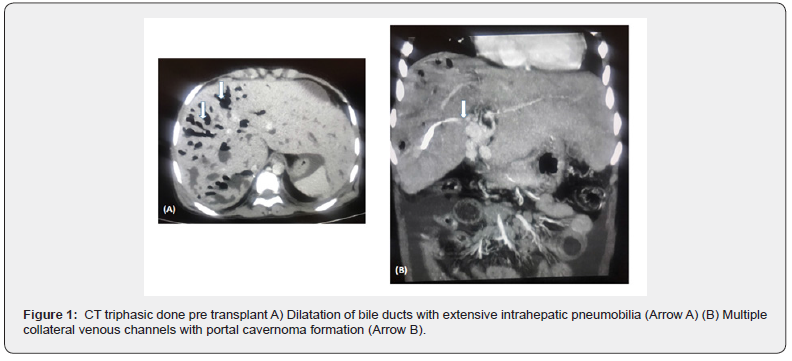
At surgery, the recipient hepatectomy was difficult as the liver was huge with pus filled pockets. The portal vein was attenuated with huge collaterals arounds the native CBD (Figure 1). The left lobe of the liver was draped over the spleen and densely adherent and could only be separated with great difficulty. She received a Modified Right Lobe graft of 480gm, with a Graft Recipient Weight Ratio (GRWR) of 1.07. There were 2 ducts and a 2 in 1 Hepaticojejunostomy was performed as the bile duct was enmeshed with huge collaterals. The Roux-en-Y hepaticojejunostomy was difficult as there were dense adhesions from previous paracentesis. The donor made an uneventful recovery
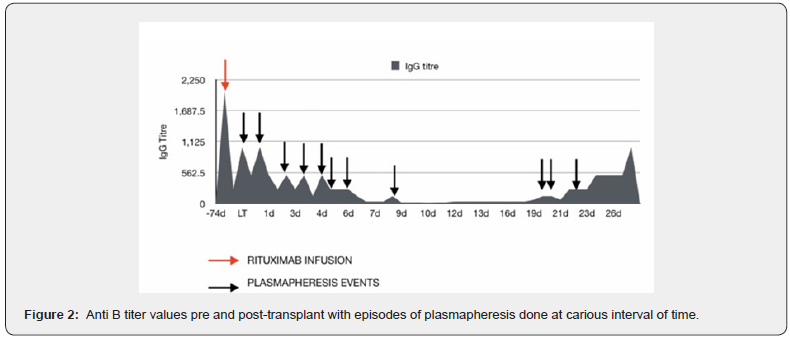
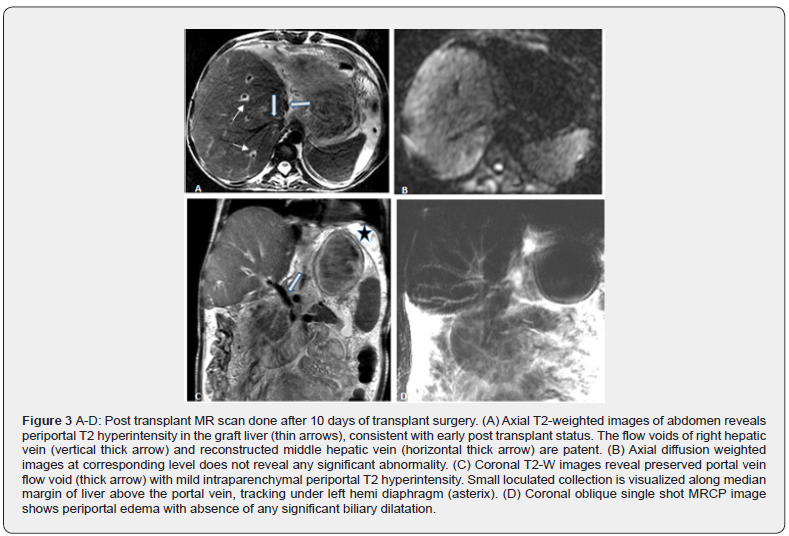
The transplanted liver functioned well in the postoperative period. However, she required multiple sessions of plasma exchanges for elevated anti B titres in the 2 weeks post-transplant (Figure 2). She was in hospital for another two weeks during which ascites was tapped. Due to a clinical suspicion of bile leak, a MRI/ MRCP was performed on POD 10 which revealed a small loculated collection in the left subphrenic region, extending from the level of porta, suggestive of a localized bile leak (Figure 3 A-D). There was mild periportal T2 hyperintensity in liver graft, consistent with early post-transplant status. The venous flow voids of right hepatic vein (RHV), reconstructed middle hepatic vein (MHV) and portal vein were maintained and there was no abnormal biliary dilatation. It was managed by subsequent percutaneous aspiration drainage alone. She was discharged on post-operative day (POD) 29 with normal liver function, on adequate immunosuppression and a healed wound. Although, her anti B titres were still 1:512, the liver function test (LFT) was normal and CD19 count was 1.9 % and CD 20 count was 1.4% therefore, it was thought that this was innocuous and unlikely to reflect humoral rejection as the LFT and portal flows were normal. During the first follow-up visit, she was well with normal LFT and a Tacrolimus level of 6.73 ng/l. Three days later on POD 35, she presented with high grade fever and mildly raised liver enzymes with normal bilirubin. An ultrasound examination was reported as being completely normal with mild ascites and good vascular flows.
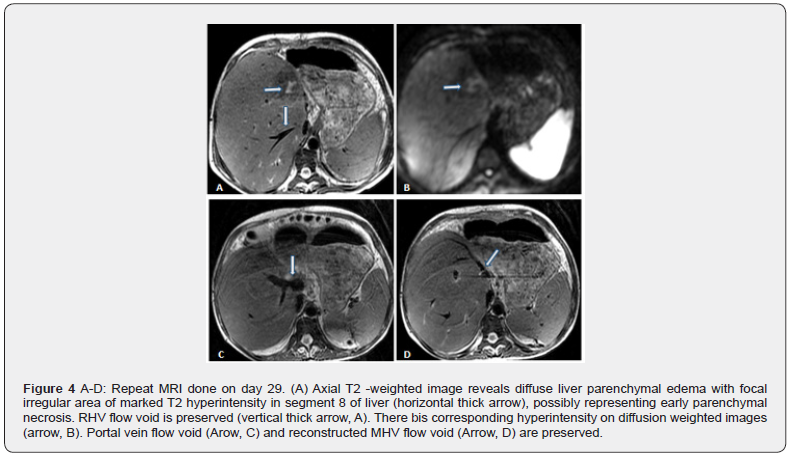
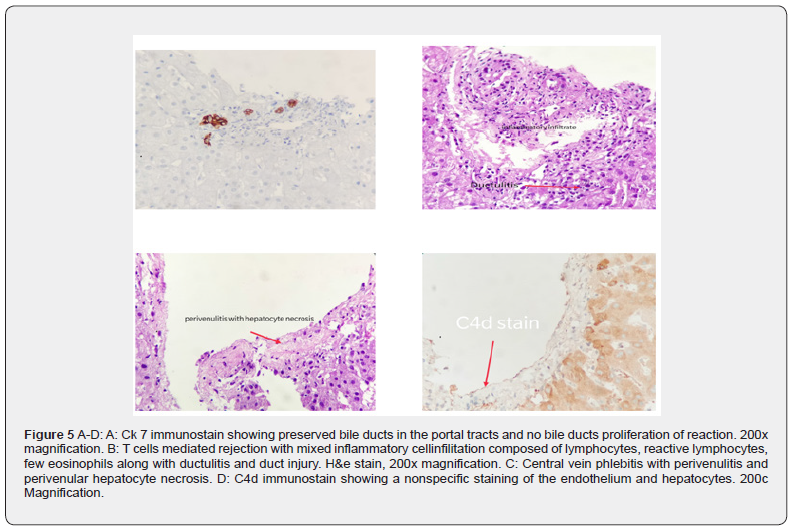
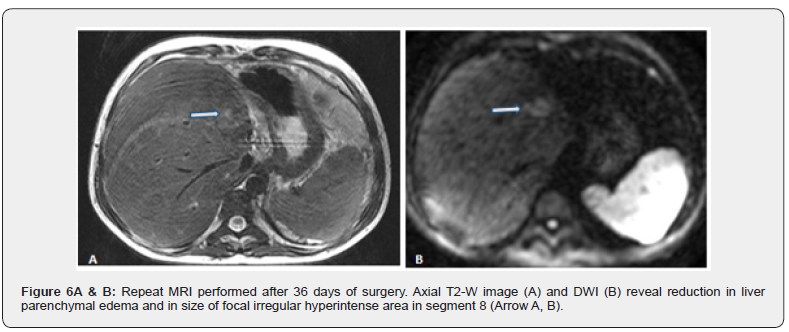
Blood cultures were sent and she was started on intravenous antibiotics and the Tacrolimus dose was increased to 5 mg three times a day. As ABO incompatible liver transplants are associated with higher biliary complications, she underwent a plain MRI/ MRCP which revealed diffuse liver parenchymal edema with a focal irregular area of marked T2 hyperintensity in segment 8 of liver measuring approximately 1.6 x 1 cm in size, possibly representing early parenchymal necrosis (Figure 4 A-D). There was a corresponding hyperintensity on diffusion weighted images. Venous flow voids were preserved and there was no abnormal biliary dilatation. Based on these findings, a liver biopsy was arranged and the patient was given five consecutive days of methylprednisolone 500 mg as intravenous bolus injections. The fever settled and liver enzymes normalised. The biopsy done on POD confirmed severe acute severe cellular rejection with rejection activity index (RAI) of 7/9. (Figure 5 A-D). Following the fourth bolus injection, she developed lower GI bleeding. A colonoscopic examination showed clear stools above the ascending colon and the mucosa was normal. Antibiotics were stopped and she was treated with oral vancomycin and metronidazole. A non contrast MR scan was repeated after eight days which showed a reduction in parenchymal oedema and in the size of the abnormal T2/ DWI hyperintense area in segment 8 (Figure 6 A-B). She was then discharged with resolution of fever and LFT. However, the anti B titres still remained high at 1: 512. The Prograf dose was given three times a day as in spite of 6 mg twice a day dose the levels remained subtherapeutic.
Discussion
There is a need for rapid diagnosis of rejection in ABO incompatible liver transplant as rejection episodes can run a fulminant course if not diagnosed and treated in time [4]. The presentation of a rejection episode particularly in the paediatric recipients can be with fever alone as a part of SIRS. At the same time sick recipients such as in our case, can have a variety of other reasons for fever and graft dysfunction [5]. They can vary from technical issues, infective complications and Small for Size Syndrome. Although attempts to combine treatment for all possible aetiologies may occasionally yield successful result, but in general a specific diagnosis is essential, should there be a need to escalate therapy. In ABO compatible LDLT, clinicians will either decrease or increase immunosuppressive dose based on the clinical impression. Depending on the response in 48 hours, the approach can be reversed in a complete “about turn” manner.
An urgent liver biopsy will give the diagnosis if done promptly. However, there are issues with Liver biopsy in LDLT settings such as an inability to repeat it frequently and the results may take 24 to 48 hours to come at the best of times. In ABO incompatible transplants, this delay may not be acceptable. Apart from the clinical presentation, multiple imaging modalities can be used to diagnose rejection by exclusion of other possibilities as discussed above [6]. Ultrasound duplex examination is the best screening tool and almost always is done as the first test. Particularly in ABO incompatible transplant, a reduction in portal volume flow by 50% combined with doubling of transaminases may suggest a diagnosis of rejection [7].
However, this is more in keeping with antibody mediated humoral rejection and has not been validated for cellular rejection. The most important role of ultrasound is to rule out vascular thrombosis. CT imaging has been reserved for confirming vascular thrombosis if suggested by duplex examination. Additionally, the thrombosis of the reconstructed Middle hepatic vein may be detected only in the CT scan which may then explain graft dysfunction if it were a new finding [6]. Occasionally, new appearance of periportal oedema may be seen with rejection. However, most often it is due to disruption of lymphatic channels in the transplanted liver. The use of MR imaging for diagnosing rejection has been limited in liver transplantation and MRCP has been used mainly to evaluate biliary complications. At our centre, in cases of graft dysfunction, ultrasound examination is carried out first and then a MRI/MRCP scan. A comprehensive noncontrast MR protocol including T2 weighted, T1 weighted, Diffusion weighted and MR cholangiography images is able to detect most of the abnormalities such as the status of graft parenchyma, the patency of portal and hepatic veins, and the integrity of the biliary system and presence of any infected collection [6]. Contrast enhanced CT angiographic study is reserved for those patients who are thought to have vascular thrombosis on ultrasound duplex examination.
If the findings on MRI/MRCP scan is satisfactory, the baseline immunosuppression is increased. In the case that we report here, the MR images suggested severe rejection with marked graft oedema and disruption of parenchyma as the vascular and biliary tree was intact and no infected collection was seen. A liver biopsy was urgently organised and methyl prednisolone bolus therapy was started. There are hardly any published literature of the use of MR imaging in diagnosing acute rejection in liver transplantation. Martino et al have described oedema in the periportal space which appears as low signal intensity on T1 weighted images and as high signal on T2 as features of acute rejection [8]. On the other hand, Lang et al have considered periportal T2 hyperintensity as being a nonspecific finding in early post-transplant period, and most likely related to postsurgical interruption in lymphatic drainage rather than being suggestive of rejection [9]. Sandrasegaran et al have suggested that ADC values may be abnormal in liver transplant recipient’s patients with graft dysfunction but lacked sufficient specificity to categorise the type of graft dysfunction. However, they studied patients who were already 3 months post transplantation and were unlikely at this stage to show graft oedema and disruption of architecture [10]. As graft swelling occurs with rejection, there is no doubt that changes in fluid content can be picked up by MR scanning as has been reported in MR scanning in cardiac transplant. Vermes et al have shown good correlation between the findings of T2 hyper intensity mapping, calculations of extracellular volume fraction and rejection in heart transplant. There is a greater need for this modality in heart transplant as there are no biomarkers for rejection and would seem a good alternative to repeated endomyocardial biopsy [11]. Similarly, Miller et al have shown that hyperintensity on T2 mapping correlated with acute cellular rejection in cardiac transplant [12]. Abouel Ghar et al. [13] has similarly reported alteration in renal allograft signal intensity on diffusion weighted MR images in cases of rejection, suggesting MR to be a promising tool in renal transplantation. It can be argued that since our case was an ABO incompatible liver transplant, the MR changes were very dramatic and therefore a diagnosis of rejection was the only possibility, particularly because the anti B titres were also high and the tacrolimus levels were low. It is possible that such dramatic changes may not be seen in the usual ABO compatible liver transplant and MR imaging can then be of limited value. However, in our experience non contrast MRI/MRCP examination has been very useful in guiding therapy in cases of graft dysfunction in LDLT as it detects biliary complications which are so much a part and parcel of this procedure more effectively. In summary, prompt management of cellular rejection is important in ABO incompatible liver transplantation. Liver biopsy is fundamental to diagnosis and treatment. However, additional imaging particularly MRI and MRC will exclude other possibilities and in some cases strongly suggest a diagnosis of acute rejection and treatment can be commenced even before the biopsy results are out.
References
- Yadav DK, Hua YF, Bai X, Lou J, Que R, et al. (2019) ABO-Incompatible Adult Living Donor Liver Transplantation in the Era of Rituximab: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract 2019: 8589402.
- Muir J (2019) Predictors of bleeding complications following percutaneous image- guided liver biopsy: A scoping review. Diagn Interv Radiol 25(1): 71-80.
- Boraschi P, Donati F, Pacciardi F, Ghinol D, Falaschi F (2018) Biliary complications after liver transplantation : Assessment with MR cholangiopancreatography and MR imaging at 3T device. Eur J Radiol 106: 46-55.
- Oh J, Kim JM (2020) Immunologic strategies and outcomes in ABO- incompatible living donor liver transplantation. Clin Mol Hepatol 26(1): 1-6.
- Shepherd RW, Turmelle Y, Nadler M, Mcdiarmid S V, Anand R, et al. (2008) Risk Factors for Rejection and Infection in Pediatric. Am J Transplant 8(2): 396-403.
- Girometti R, Pancot M, Como G, Zuiani C (2017) Imaging of liver transplantation. Eur J Radiol 93: 295-307.
- H Sugimoto, T Kaneko, Y Marui, S Inoue, T Seo, T, et al. (2001) Reversal of portal flow after acute rejection in living-donor. J Hepatobiliary Pancreat Surg 8(6): 573-576.
- Michele Di Martino, Massimo Rossi, Gianluca Mennini, Fabio Melandro, Michele Anzidei (2016) Imaging follow-up after liver transplantation Br J Radiol 89(1064): 20151025.
- Philipp Lang, Peter Schnarkowski, Stephan Grampp, Cornelis van Dijke, Alexander Gindele RS et al. (1995) Liver Transplantation: Significance of Periportal Collar on MRI. J Comput Assist Tomogr 19(4): 580-585.
- Sandrasegaran K, Ramaswamy R, Ghosh S, Tahir B, Akisik FM, et al. (2011) Diffusion-weighted MRI of the transplanted liver. Clin Radiol 66(9): 820-825.
- Vermes E, Pantaléon C, Auvet A, Cazeneuve N, Machet MC, et al. (2018) Cardiovascular magnetic resonance in heart transplant patients: diagnostic value of quantitative tissue markers: T2 mapping and extracellular volume fraction, for acute rejection diagnosis. J Cardiovasc Magn Reason 20(1): 59.
- Miller CA, Fildes JE, Ray SG, Doran H, Yonan N, et al. (2013) Non-invasive approaches for the diagnosis of acute cardiac allograft rejection. Heart 99(7): 445-453.
- Refaie HF, Refaie AF (2012) Role of diffusion-weighted MRI in diagnosis of acute renal allograft dysfunction: A prospective preliminary study. Br J Radiol 85(1014): e206-11.






























