The Impact of COVID-19 Pandemic on Living Donor Liver Transplantation: An Integrated Mitigation Strategy with Successful Outcome
Amr Abdelaal1, Amr El-Fouly2, Ahmed Mukhtar3, Khalid Ewida4, Mostafa Alfishawy5, Mostafa Abdo4, Mohamed El-Adawy4, Ahmed Abdelshafy A4, Mohamed Alaa Maklad6, Tawhida Abdel-Ghaffar4, Neha Parekh R6, Ayat ElSherif6 and Kareem Abu-Elmagd6*
1Ain-Shams University, Egypt
2Helwan University, Egypt
3Cairo University, Egypt
4Air Force Specialty Hospital (AFSH), Egypt
5Center of Infectious Disease Consultant and Academic Research of Egypt (IDCARE), Egypt
6Department of General Surgery, Cleveland Clinic Foundation, Cleveland, OH
Submission:April 27, 2021; Published:May 04, 2021
*Corresponding author: Kareem Abu-Elmagd, Professor of Surgery, Case western Reserve University, Director , Center For Gut Rehabilitation and Transplantation (CGRT), Cleveland Clinic Foundation, Cleveland, OH, USA
How to cite this article: Amr A, Amr E-F, Ahmed M, Khalid E, Mostafa A, et al. The Impact of COVID-19 Pandemic on Living Donor Liver Transplantation: An Integrated Mitigation Strategy with Successful Outcome. Adv Res Gastroentero Hepatol, 2021; 16(5): 555949. DOI: 10.19080/ARGH.2021.16.555949.
Abstract
With declaration of the pandemic, 339 patients were managed at a single LDLT center comprising the reported population. Of these, 315(93%) were pre-pandemic recipients and 24(7%) were active candidates. From the outset, integrated national, regional and hospital-based mitigation strategies were promptly defined and implemented. To measure the impact of the outbreak and adjust for non-COVID-19 covariates, a case-time-control group was identified. With 9-month follow-up, SARS-CoV-2 was diagnosed in 3(1%) pre-COVID-19 LDLT recipients and 2(8%) active-candidates with respective mortality of 33% and 100%. With 8(33%) total waiting-list mortalities, the remaining 16(67%) underwent urgent LDLT. Compared to the case-time-control group, the pandemic significantly (p<0.001) reduced transplant activity and increased (p=0.01) waiting-list mortality. With transplantation, none of the donors, recipients, or healthcare providers developed COVID-19. Preoperative screening detected SARS-CoV-2 in 1(6%) donor advancing to donation after negative RT-PCR. Transplantation achieved similar (p=0.38) 6-month survival among study (92%) and case-time-control (84%) recipients. Hospital stay was shorter (p=0.006) with fewer (p=0.11) readmissions and no primary infectious complications among study recipients. To our knowledge, this is the first study to prospectively assess COVID-19 impact on LDLT and describe measures allowing full return of transplant activities. With integrated COVID-19 mitigation strategies, LDLT is safe and achieves excellent outcome.
Keywords: Live donor; Liver transplantation; Covid-19 infection; Mitigation Strategy
Abbreviations: AFSH: Air Force Specialty Hospital; CRP: C-Reactive Protein; COVID-19: Coronavirus Disease 2019; HCC: Hepatocellular Carcinoma; ICU: Intensive Care Unit; IGRA: Interferon Gamma Release Assay; IL-6: Interleukin-6; LOS: Length of Hospital Stay; LDLT: Living Donor Liver Transplantation; MELD: Model For End-Stage Liver Disease; MMF: Mycophenolate Mofetil; PPE: Personal Protective Equipment; PVT: Portal Vein Thrombosis; RT-PCR: Reverse Transcriptase Polymerase Chain Reaction; SARS CoV-2: Severe Acquired Respiratory Syndrome Coronavirus 2
Introduction
In a cohesive and coordinated fashion, the national and international transplant communities swiftly responded to the unprecedented Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic [1-8]. The major challenge was how to strike the balance between the prohibitive risk of transmitting the potentially lethal SARS-CoV-2 and the expected high mortality among organ failure patients without transplantation [4]. Most of the world experts advocated temporary halt or reduction of solid organ transplantation with a staged approach gauged by the surge of the infection, resource availability, and risk tolerance [1-9]. Meanwhile, mitigation strategies were proposed with a global perspective to successfully navigate the pandemic and ultimately fully restore transplant activities [1-13].
With identification of the first SARS-CoV-2 infected case at Cairo airport, the Egyptian living donor liver transplant (LDLT) activities were halted at all 15 national transplant centers except the Air Force Specialty Hospital (AFSH) [14]. With the country’s first reported LDLT performed in 2001, the pre-COVID average number of LDLT was 275 per year [15]. The decision to continue transplant activity at AFSH stemmed from the high demand for LDLT with the lack of a national platform to implement the 2010 parliament approval of deceased donation [15]. Accordingly, an institution-based mitigation strategy, along with strict national and regional policies, was promptly implemented in alignment with national and international transplant society recommendations (2, 3, 6-8). A resource utilization plan was also outlined with a distinctive clinical care path. The utilized immunosuppressive protocol continued to be guided by the welldocumented immunologic privileges of the liver [16,17].
During the early phase of the COVID-19 pandemic, several scientific articles were urgently published including expert opinions, transplant society recommendations, and worldwide internet-based survey data [1-33]. In addition, a few transplant centers from the pandemic red zones published their early experience with COVID-19 infected recipients and outcome after a small number of transplantations [18-33]. To the best of our knowledge, this is the first prospective case-time-control study that objectively assesses the multilayered impact of COVID-19 outbreak on LDLT. New insights are also discussed in the milieu of the herein utilized mitigation strategies.
Materials and Methods
Study design
The study was conceptualized soon after the announcement of the first SARS-CoV-2 infected case in Egypt [14,34]. The central core of the study design was adoption of a comprehensive mitigation strategy including RT-PCR testing as recommended by many infectious disease experts and transplant societies [2,3,6-8]. The reported population included recipients of prior LDLT and active transplant candidates including those who underwent LDLT during the pandemic. Donors and healthcare providers were also part of the study population.
To assess the impact of the pandemic on transplant activity and other important outcome measures, a case-time-control group was identified adjusting for exposure time trends and covariates other than COVID-19. Accordingly, the utilized methodology reduces confounding when both the exposure and outcome are time varying and have been measured at several time points for each individual.
Patient population
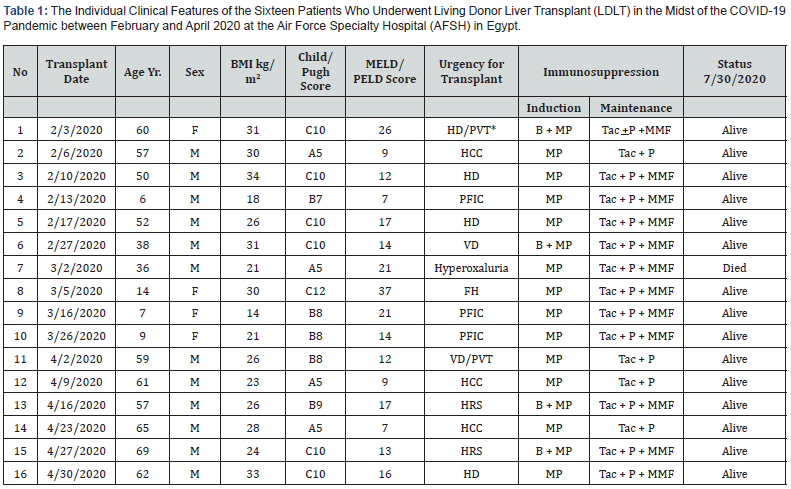
BMI: Body Mass Index; *Suspected Early Endometrial Carcinoma; HD: Hepatic Decompensation; PVT: Portal Vein Thrombosis; B: Basiliximab; MP: Methyl Prednisolone; Tac: Tacrolimus; P: Prednisone; MMP: Mycophenolate Mofetil; HCC: Hepatocellular Carcinoma; PFIC: Progressive Familial Intrahepatic Cholestasis; VD: Vascular Decompensation; HRS: Hepatorenal Syndrome
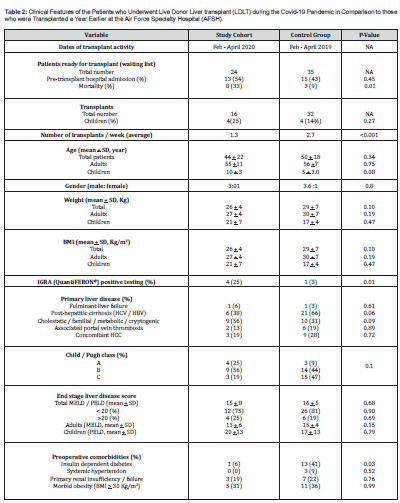
NA: Not Applicable; BMI: Body Mass Index; HCV: Hepatitis C Virus; HBV: Hepatitis B Virus; HCC: Hepatocellular Carcinoma; MELD: Model for End Stage Liver Disease; PELD: Pediatric End-Stage Liver Disease Model; IGRA: Interferon Gamma Release Assay
This report included a total of 339 patients. Of these, 315(93%) were pre-COVID-19 established LDLT recipients and followed at the AFSH transplant center since the 2015 inception of the program. The remaining 24(7%) were liver failure patients awaiting LDLT comprising the study cohort. With no single patient refusing transplantation because of the pandemic, 16(67%) underwent LDLT between February 1st and April 30th, 2020. All patients had liver failure-associated life-threatening complications dictating the urgent need for hepatic replacement in the absence of an active national deceased donor program [15].
Patients transplanted during the pandemic had an age range of 6 to 69 years. Non-hepatitic cirrhosis and associated comorbidities were documented in more than half of the cases. Despite a relatively low MELD score in some of the study patients, life-threatening complications including variceal bleeding, hepatic encephalopathy, intractable ascites, renal failure, and intra-abdominal infections were common with the need for hospitalization prior to transplantation. The individual patient complexity and associated malignancy including hepatocellular carcinoma (HCC) are shown in Table-1.
The case-time-control group included a total of 35 patients who were potential candidates for LDLT one year prior to the pandemic (February 1st - April 30th, 2019). Of these, 32(91%) were transplanted during that time period with clinical features collated in Table-2. Most were adult males with an age range of 2 to 71 years. The primary liver disease was viral hepatitis cirrhosis in more than half of the cases with associated comorbidities including portal.
Mitigation strategy
The adopted strategy consisted of three integrated tiers: government rules, ministry of health measures, and AFSHtransplant center protocols. With the timeline depicted in figure 1, most of the universal measures including wearing a mask, social distancing, and hand sanitization were adopted as recommended by the World Health Organization and Centers for Disease Control [1-12]. All international flights, worship facilities, and education institutes were suspended with an enforced countrywide nighttime curfew. All travelers from the national and international COVID-19 hot zones were quarantined for 14-days utilizing isolated healthcare facilities. During the early phase of the pandemic, COVID-19 RT-PCR testing was limited to symptomatic patients and healthcare providers.
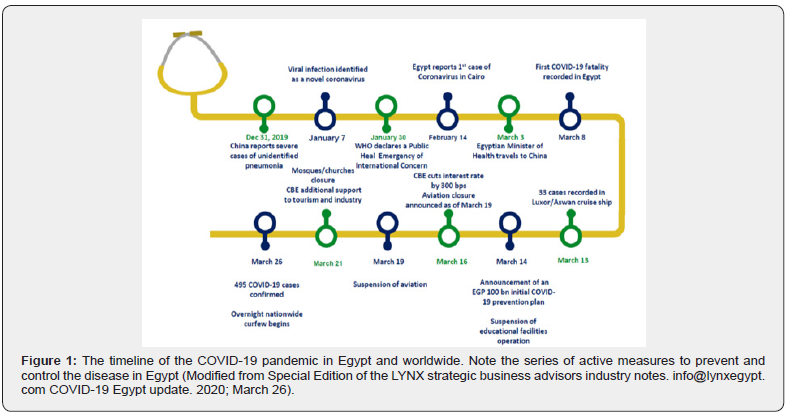
The ministry of health optimized frontline healthcare services and designated 23the country exclusively for the isolation and management of SARS-CoV-2 infected cases including liver transplant recipients. These hospitals were fully equipped with nasopharyngeal RT-PCR testing and a multispecialty team armed with personal protective equipment (PPE). The treatment protocol evolved as a result of the worldwide growing experience with antiviral, anti-inflammatory, and anticoagulation therapy. The AFSH was designated from the outset as a COVID-free hospital and only life-saving procedures were offered with no visitors allowed at any time. All hospital employees and patients were periodically screened for COVID-19 infection utilizing the universally adopted questionnaire. Potentially high risk asymptomatic and symptomatic individuals underwent RT-PCR testing. A two-week quarantine was required for the PCR-positive asymptomatic individuals with referral of the symptomatic patients to a designated COVID-19 hospital.
The transplant center was provided with two operating rooms, an intensive care unit, and inpatient ward fully supplied with PPE. Admittance to all liver transplant facilities including outpatient clinic was limited to transplant caregivers with no cross trafficking. As such, transplant activities were streamlined to eliminate the potential risk of COVID-19 transmission.
Within the transplant center, a COVID-19 questionnaire was also provided to all candidates, potential donors, transplant patients, and healthcare workers to assess individual risk stratification. A 14-day quarantine and molecular testing with RTPCR were required for any healthcare personnel with history of exposure or symptoms suggestive of COVID-19 infection. COVID-19 preventive educational materials and virtual seminars were provided with periodic updates. Potential donors were advised to stay home and recipients to be isolated, if not hospitalized, prior to transplant.
Transplant activity
With an expected high 3-month mortality, the urgent need for transplantation was determined by a multidisciplinary team. The committee included transplant hepatologists, surgeons, infectious disease experts, an ethicist, and a social worker. The leading indication for transplant was rapid deterioration in clinical condition including those with fulminant hepatic failure, high MELD score, life-threatening complications, and HCC.
The criteria to suspend or gradually increase transplant activity were established from the outset. Immediate suspension was warranted with development of confirmed case(s) among patients admitted to the transplant unit or healthcare providers. Conversely, gradual increase in activity was anticipated when the national infectivity rate declined in the absence of a second wave. Equally important was the continual achievement of a national recovery rate higher than 95%.
Potential donors and recipients with no clinical symptoms and low questionnaire risk stratification were admitted 24-48 hours prior to transplant. CT chest, arterial blood gas, and deep nasopharyngeal swabs for SARS-CoV-2 RT-PCR testing were mandatory for both donors and recipients. Baseline inflammatory markers including C-reactive protein (CRP) titer and serum procalcitonin level were also measured. With negative testing, the date of transplant was confirmed by teleconference. Timing of the next transplant was dependent on the anticipated date of ICU discharge of both donor and recipient.
Transplant protocol
All donors and recipients were Egyptian citizens. Living donation followed national and international regulations with strict supervision by multiple Egyptian authorities [15,35,36]. The geographic distribution and management protocol were the same since the 2015 inception of the program [35,36]. All transplants were ABO compatible with immunologic assays performed when clinically indicated.
Induction therapy was used for all patients with a steroid taper. Basiliximab was utilized in selected cases. Methyl prednisolone was administered intravenously in a 500 mg dose at time of transplant with 1mg/Kg/day for the first 3 postoperative days followed by 0.5 mg/kg/day until postoperative day 6 [15,35]. Basiliximab was given intravenously in a dose of 20 mg on 1st and 4th post-transplant day. The antibody was utilized as a calcineurininhibitor renal-sparing agent in the presence of chronic renal impairment, hepatorenal syndrome, and high disease gravity [37,38].
Maintenance immunosuppression was tacrolimus–steroid based. During the first 6 postoperative weeks, gradual weaning of the initial 20 mg daily steroid dose was adopted in all patients with the addition of mycophenolate mofetil (MMF) in non-HCC patients. The targeted 12-hour tacrolimus trough level was 8-10 ng/ml. After the 6th week landmark, everolimus was added to the steroid-spared regimen particularly for patients with HCC or renal impairment. With the combined regimen, the targeted trough level for each drug was 3-5 ng/ml. Most rejection episodes were treated with augmented steroid therapy.
With suspected or confirmed SARS-CoV-2 diagnosis, reduction of immunosuppression was guided by clinical, respiratory, and hemodynamic status. Discontinuation of MMF with 50% reduction of the tacrolimus daily dose was adopted in cases with mild to moderate disease. In patients with respiratory failure and hemodynamic instability, tacrolimus was discontinued and high dose intravenous daily steroids were given along with systemic anticoagulation and other anti-viral therapeutics.
Postoperative management
With the surge of the pandemic, the role of transplant infectious disease specialists became central to patient care. Health education with daily update on COVID-19 was delivered to patients and healthcare providers. All parties were disciplined and abided with the infection control regulations.
Inpatient and outpatient care continued to be multidisciplinary during the pandemic. Outpatient activities were strategically modified with more virtual visits, frequent follow-up phone calls, and limited laboratory visits. A COVID-free outpatient clinic was dedicated to patients in need of in-person visits particularly those with recent transplant.
Management of COVID-19 infection was similar to what has been described elsewhere [39,40]. No chemoprophylaxis was recommended to healthcare personnel, recipients or donors. Patients and healthcare providers with suspected or confirmed COVID-19 infection were transferred to the nearest dedicated hospital for isolation and prompt medical care. The daily management of these patients was conducted via a daily teleconference between the transplant team and the COVID-19 dedicated hospital staff.
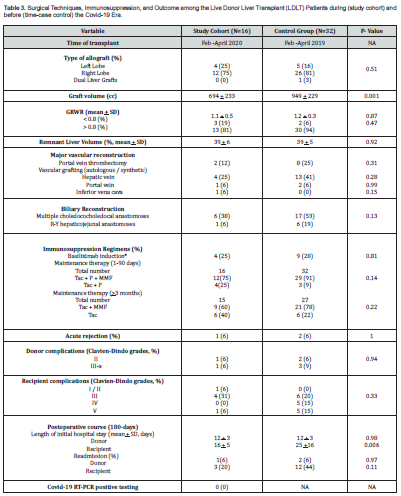
*All recipients received 500mg intravenous methyl prednisolone; NA: Not Applicable; BMI: Body Mass Index; HCC: Hepatocellular Carcinoma; MELD: Model for End Stage Liver Disease; PELD: Pediatric End-Stage Liver Disease Model; GRWR: Graft Recipient Weight Ratio; Tac: Tacrolimus; P: Prednisone; MMP: Mycophenolate Mofetil
Statistical analysis
Case-time-control methodology was used to identify a control group to adjust for exposure time trends and additional covariates other than COVID-19. This statistical design allows control for confounding by time-varying covariates, while controlling for all time-stationary characteristics of the study participants [41].
The prospectively collected data for each of the study and control patients were retrieved from the liver transplant electronic database after approval of the AFSH Institutional Review Board. Individual data was collated and summarized as mean + standard deviation for continuous and percentage for categorical variables. Clavien-Dindo classification was utilized to grade postoperative complications [42]. Group differences were examined with the unpaired t-test for continuous and Person chi-squared or Fisher exact tests for nonparametric variables. Survival was calculated with Kaplan-Meier product limit method and log-rank test was used for group comparison. All events were computed as of October 15th, 2020.
Results
Transplant activity and waiting-list mortality
The pandemic significantly (p <0.001) reduced total and weekly number of LDLT with a higher (p=0.01) waiting-list mortality compared to the control era (Table 2). Of the 8(33%) pandemic candidate mortalities, 2(25%) died of COVID-19- associated acute myocardial infarction and systemic inflammatory response. The remaining 6 died of variceal hemorrhage (n=2), spontaneous bacterial peritonitis (n=2) and fungal/bacterial pneumonia (n=2). The causes of the 3(9%) case-time-control candidate mortalities were spontaneous bacterial peritonitis (n=2) and variceal hemorrhage (n=1).
Transplantation
Descriptive analysis: The clinical features of both the study and case-time-control transplant recipients are summarized in table 2. The study cohort had a higher (p>0.05) percentage of children, fulminant liver failure, non-hepatitic cirrhosis, and > 20 MELD/PELD scores compared with the control group. In contrast, the control group experienced a higher percentage of HCC and preoperative comorbidities particularly insulin dependent diabetes (p=0.03). Interferon Gamma Release Assay (IGRA) was positive at a significantly (p=0.01) higher rate among the study cohort.
Surgical techniques:
The type of utilized allograft and complexity of the transplant procedure were similar during both eras (Table 3). However, more challenging vascular and biliary reconstructions were required for the control group with one example of a dual allograft. With a higher percentage of adult recipients, mean g raft volume was significantly (p=0.001) higher among the control group. Graft recipient weight ratio and donor remnant liver volume were similar among both groups with respective p values of 0.87 and 0.92.Immunosuppression: With no intention to modify the regimen, the study cohort received a lower level of maintenance immunosuppression. The control group received a higher rate of triple and double drug therapy during the first 90 post-transplant days and thereafter, respectively (Table 3). Albeit, both groups experienced the same low rate of acute rejection during the 9-month follow-up period.
Hospital Course: With similar mean values, the average length of hospital stay (LOS) was 10 (range:8-18) days for the study and 14 (range:6-20) days for the control donors (Table 3). However, the recipient mean LOS was significantly (p=0.006) shorter among the study compared to the control group with a respective maximal stay of 28 and 83 days. During the 9-month follow-up period, the frequency of donor readmission was 6% in each group with no significant (p=0.11) difference in recipient readmission; 20% for the study and 44% for the control group.
Donor complications: A total of 2(12%) study and 5(15%) control donors developed postoperative complications with no significant (p=0.94) difference between both groups (Table 3). Wound infection occurred in 1 study and 2 control donors with localized intra-abdominal biliary collection in the remaining 1 and 3 donors, respectively. All were successfully treated with intravenous antibiotics and bedside or percutaneous drainage. Recipient complications: Study recipients experienced less (p=0.33) postoperative complications compared to the control group with an overall incidence of 38% and 50%, respectively (Table 3). Concerning the study group, the 6 morbid cases developed 9 postoperative complications; 8(89%) technical and 1(11%) metabolic. Technical complications were biliary (n=5), sub-hepatic hematoma (n=1), and wound dehiscence (n=1). All were successfully treated with endoscopic, radiologic, surgical, or medical intervention. The remaining case succumbed to congestive heart failure. There was no single example of primary microbial infection.
The 16 morbid control recipients experienced 19 complications; 8(42%) technical, 6(32%) infectious, and 5(26%) metabolic. Technical complications were 1(13%) vascular and 7(88%) biliary. Infectious morbidities were multi-resistant systemic gram negative sepsis (n=4), pneumonia (n=1), and fungemia (n=1). Treatment was successful in all but 5 recipients.
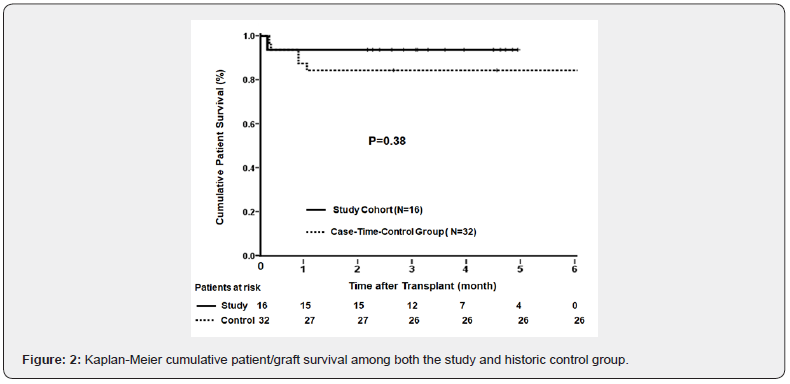
Survival: During the 9-month observation period, 1(6%) of the 16 study recipients died 4 days after transplant of congestive heart failure with preexisting cardiomyopathy and end-stage chronic renal failure. Of the 32 case-time-control recipients, 5(16%) died within 5 to 27 days of transplant due to portal vein thrombosis-associated graft failure (n=1), progressive multiple leucoencephalopathy (n=1), and systemic sepsis (n=3). The 6-month cumulative survival was 92% for the study and 84% for the control recipients (Figure 2). The difference was not significant (p= 0.38) with a hazard ratio of 2.4.
SARS-CoV-2 infection
COVID-19 infection was confirmed by RT-PCR testing in 2(8%) of the 24 active candidates. The two community-infected patients were 48 and 61 years old with Child C cirrhosis. Both patients died, with history of ischemic heart disease in one, despite comprehensive management including optimal respiratory support, high dose steroids, and broad spectrum antibiotics. However, no anticoagulation treatment was given with the lack of an effective antiviral and anti-IL6 therapy at that time.
Preoperative RT-PCR screening was positive in 1(6%) of the 16 potential donors. The community-infected asymptomatic donor was quarantined for 14 days and transplant was performed after two consecutive negative RT-PCR tests one week apart. COVID-19 was suspected in another potential donor with elevated liver enzymes and low lymphocytic count. Despite RT-PCR was postponed. Three weeks later, transplantation was performed after normalization of liver enzymes with restored lymphocytic count, normal pulmonary imaging, and reconfirmed negative RTPCR.
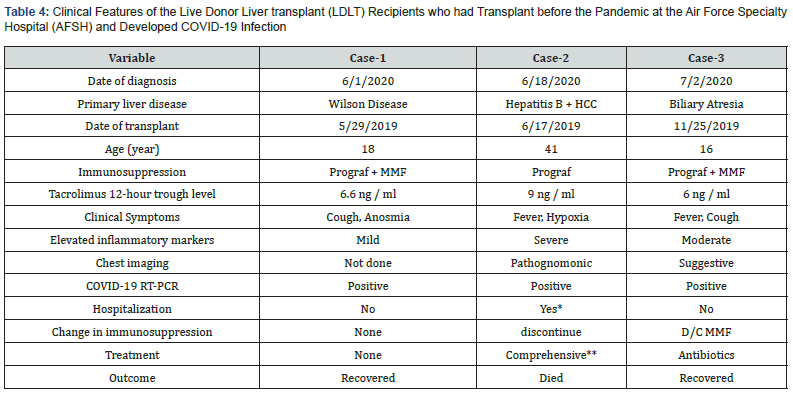
*At the COVID-19 dedicated hospital, **Zithromax, hydroxychlorquine, zinc, vitamin C, anticoagulation. HCC: Hepatocellular Carcinoma, MMF: Mycophenolate Mofetil
During the 9 month study period, none of the donors and healthcare personnel developed symptoms suggestive of SARSCoV- 2 infection or tested positive with RT-PCR. Also, all of the patients transplanted during the pandemic continued to be free of SARS-CoV-2 infection with negative RT-PCR. Of the pre-COVID-19 LDLT survivors, 4 experienced clinical symptoms suspicious of COVID-19 infection 7 to 15 months from date of transplant. None of these were case-time-control recipients with 3 testing positive giving an overall infection rate of 1%.
Individual clinical features of the 3 COVID-19 infected recipients are summarized in table 4. Increases in liver enzymes, CRP, and other inflammatory markers were moderate in the two young recipients with the adolescent developing symptoms soon after the mother was diagnosed with COVID-19. Both young recipients did not require hospitalization and were managed conservatively with reduction of immunosuppression in one. The disease was overwhelming in the 41-year old recipient with accelerated respiratory failure and hemodynamic instability. Despite comprehensive management including anticoagulation therapy, the patient died 4 days later with a COVID-19-associated mortality of 33%.
Discussion
The impact of the COVID-19 pandemic on transplantation has yet to be fully elucidated. The currently available published data are limited to clinical surveys and a few case series [2-13,18-33]. To the best of our knowledge, this is the first prospective casetime- control study that provides evidence-based assessment of the multidimensional impact of COVID-19 on LDLT and allied healthcare workers. The results of an integrated mitigation strategy are highlighted with the encouragement to eventually restore pre-pandemic transplant activities.
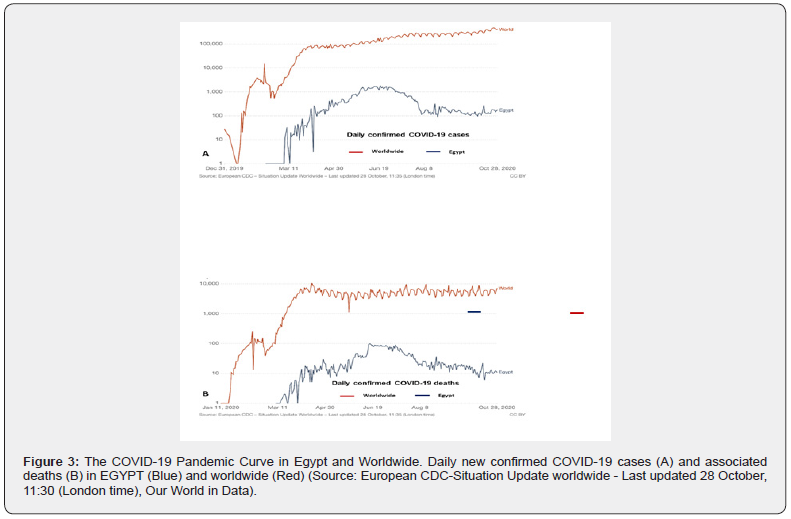
With declaration of the pandemic, high anxiety and uncertainty were born restricting transplant activities in Egypt and worldwide. During the study period, all of the Egyptian transplant centers except AFSH halted transplant activity. Worldwide, the level of restriction was gauged by the geographical prevalence and fatality of COVID-19, availability of healthcare resources, and risk tolerance (Figure-3) [1-8,43]. Of great fear, was transmitting the lethal pathogen to healthy donors, vulnerable patients, and healthcare workers. Such an ethical dilemma has been more significant in countries where LDLT is the only therapeutic modality available for patients with liver failure. As validated herein, limiting transplant activity to patients with progressive hepatic decompensation and life-threatening complications can significantly increase waiting-list mortality.
Our integrated three tier mitigation strategy allowed for performance of LDLT without evidence of community or hospitalacquired viral infection. None of the study donors, recipients, and healthcare providers developed COVID-19 infection. All received the recommended universal preventive measures and preoperative screening including molecular testing [1-12]. With positive SARS-CoV-2 RT-PCR, two consecutive negative tests were required before transplantation with a similar approach being recently advocated [4,9,10,43]. One of the novel features of this study is redesigning the workflow within a dedicated COVID- 19-free hospital based program. With strict adherence to these proactive measures, transplant programs have the potential to continue operating despite the unpredictability of the pandemic.
This study supports the worldwide reported vulnerability of liver transplant candidates and recipients to COVID-19 infection with associated high fatality [2,18,20-22]. With controversies concerning the role of immunosuppression, disease severity was reported to be higher among recipients with major comorbidities including obesity, diabetes, and cardiovascular diseases [43]. Fatality was driven by older age, higher sequential organ failure assessment score, and dimer > 1 mcg/ml on admission [20,43]. It remains to be seen if the protective effect of childhood BCG vaccination in the non-western population translates to transplant recipients [44]. Nonetheless, preemptive screening of the seemingly high-risk liver transplant candidates and recipients is strongly recommended with management strategy steered by up-to-date best practice guidelines [30,43].
Optimal management of COVID-19 has yet to be well established awaiting the results of several clinical trials including monoclonal antibodies and the mRNA vaccine [43]. With the anticipated expedited government approval, the clinical availability of these novel therapeutic modalities will ultimately control the pandemic [45]. Until then, low risk transplant recipients with mild symptoms can be managed at home with close monitoring for clinical deterioration. With moderate to severe disease, patients should be hospitalized and receive prophylactic anticoagulation therapy to ameliorate COVID-19- associated systemic microvascular thrombosis. Broad spectrum antibiotics should be given to patients with suspected pneumonia or sepsis and those who require supplemental oxygen or mechanical ventilation may benefit from dexamethasone therapy [2,21,24,26,27]. Remdesvir is recently approved by the FDA and convalescent plasma is currently classified as an investigational product [46]. A few case series have also shown some benefits of the IL-6 inhibitor, tocilizumab [43].
Currently, there is no evidence-based recommendation for the management of immunosuppression during the pandemic [27,43]. In this study, the same pre-COVID-19 induction and maintenance immunosuppressive protocols were used for patients transplanted during the pandemic. However, there was an unintentional tendency to reduce the level of maintenance therapy. In recipients diagnosed with coronavirus infection, immunosuppression was modified in those with ongoing symptoms and totally withdrawn with severe illness. Similar approaches were advocated by most centers across the world [2,18,27,43]. It remains to be seen if immunosuppression aggravates COVID-19 infection, alleviates the triggered inflammatory innate immune response, or suppresses the response to future vaccination [47].
This study highlights some important unforeseen observations. The implemented mitigation strategies including strict universal infectious control measures reduced risk of hospital acquired infection with shortening of hospital stay. Previously infected individuals with negative RT-PCR assay can be safely considered for living or deceased donation. Telehealth came to stay along with continual attempts to optimize comorbidities and immunosuppression. Despite the small sample size and short follow-up, these results underscore the foremost role of novel clinical practice dictated by the pandemic in improving outcome and value of healthcare.
Conclusion
LDLT can be safely performed during the COVID-19 pandemic in both the non-western and western countries. The documented herein outcomes allowed most Egyptian LDLT centers to resume transplant activities in a stepwise fashion. The worldwide clinical availability of effective vaccines along with integrated mitigation strategies will hopefully permit the full return of pre-pandemic transplant activities with successful outcome.
References
- Kumar D, Manuel O, Natori Y, Hiroto Egawa, Paolo Grossi, et al. (2020) COVID-19: A global transplant perspective on successfully navigating a pandemic. Am J Transplant 20(7): 1773-1779.
- Boyarsky B, Po-Yu Chiang T, Werbel W, Christine M Durand, Robin K Avery, et al. (2020) Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant 20: 1809-1818.
- Lau G, Ward JW (2020) Synthesis of liver association recommendations for hepatology and liver transplant care during the COVID-19 pandemic. Clin Liv Dis 15(5): 204-209.
- Esagian SM, Ziogas IA, Giannis D, Muhammad Hayat H, Nahel Elias, et al. (2020) Challenges in abdominal organ transplantation during the COVID-19 pandemic. Front Med 287: 1-8.
- Agopian V, Verna E, Goldberg D (2020) Changes in liver transplant center practice in response to coronavirus disease 2019: Unmasking dramatic center-level variability. Liver Transplant 26(8): 1052-1055.
- Ritschl PV, Nevermann N, Wiering L, Helen Wu, Philipp Moroder, et al. (2020) Solid organ transplant programs facing lack of empiric evidence in the COVID-19 pandemic: A by-proxy society recommendation consensus approach. Am J Transplant 20(7): 1826-1836.
- Lau G, Sharma M (2020) Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol International 14: 415-428.
- Fix OK, Hameed B, Fontana RJ, Ryan M Kwok, Brendan M McGuire, et al. (2020) Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatol. 72(1): 287-304.
- Galvan NTN, Moreno NF, Garza JE, Susan Bourgeois, Bhamidipati Murthy, et al. (2020) Donor and transplant candidate selection for solid organ transplantation during the COVID-19 pandemic. Am J Transplant 20: 3113-3122.
- Shah MB, Lynch RJ, El-Haddad H, Brianna Doby, Diane Brockmeier, et al. (2020) Utilization of deceased donors during a pandemic: Argument against using SARS-CoV-2-positive donors. Am J Transplant 20(7): 1795-1799.
- Chung SJ, Tan EK, Kee T (2020) Practical considerations for solid organ transplantation during the COVID-19 global outbreak: The experience from Singapore. Transplant Direct 6: 1-9.
- Mujtaba MA, Kueht M, Merwat S, Hussain S, Fair J, et al. (202) Organ transplantation during COVID-19 pandemic: Making the best patient care decision. Am J Transplant 20(11): 3259-3260.
- Verma S, Agarwal S, Chikkala BR, Dey R, Singh S, et al. (2020) Living donor liver transplants for sick recipients during COVID-19 pandemic-An experience from a tertiary center in India. Am J Transplant 20(11): 3257-3258.
- (2020) Special Edition of the LYNX strategic business advisors industry notes.
- Amer KE, Marwan I (2016) Living donor liver transplantation in Egypt. Hepatobil Surg Nutr 5(2): 98-106.
- Calne RY, Sells RA, Pena JR Jr, Davis, DR, Millard PR, et al. (1969) Induction of immunological tolerance by porcine liver allografts. Nature 223: 472-476.
- Starzl TE (2008) Immunosuppressive therapy and tolerance of organ allografts. N Engl J Med 358(4): 407-411.
- Polak WG, Fondevila C, Karam V, Adam R, Baumann U, et al. (2020) Impact of COVID-19 on liver transplantation in Europe: Alert from an early survey of European liver and intestine transplantation association (ELITA) and European liver transplant registry (ELTR). Transpl Int 33(10): 1244-1252.
- Hong H-L, Kim S-H, Choi DL (2020) A case of coronavirus disease 2019-infected liver transplant donor. Am J Transplant. 20(10): 2938-2941.
- Nacif LS, Zenini LY, Waisberg DR, Pinheiro RS, Galvão F, et al. (2020) COVID-19 in solid organ transplantation patients: A systematic review. Clinics (Sao Paulo) 75: e1983.
- Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, et al. (2020) COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant 20(7): 1800-1808.
- Kates OS, Fisher CE, Stankiewicz-Karita HC, Shepherd AK, Church EC, et al. (2020) Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplantation in the United States. Am J Transplant 20(7): 1885-1890.
- Nair V, Jandovitz N, Hirsch JS, Nair G, Abate M, et al. (2020) COVID-19 in kidney transplant recipients. Am J Transplant 20(7): 1819-1825.
- Liu B, Wang Y, Zhao Y, Shi H, Zeng F, et al. (2020) Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant 20(7): 1891-1895.
- Gao F, Zheng K, Gu JY, George J, Ming-Hua Z (2020) COVID-19 and liver transplantation; lessons learned from three reported cases. Transpl Infect Dis 22(4): e13335.
- Kolonko A, Dudzicz S, Wiecek A (2020) COVID-19 infection in solid organ transplant recipients: A single-center experience with patients immediately after transplantation. Transpl Infect Dis 23(1): e13381.
- Hoek RAS, Manintveld OC, Betjes MGH, Hellemons ME, Seghers L, et al. (2020) COVID-19 in solid organ transplant recipients: A single center experience. Transpl Int 33(9): 1099-1105.
- Rhee Y, Chan EL, Eswaran SL, Aloman C, Hertl M, et al. (2020) Fatal COVID-19 in a patient with end-stage liver disease wait-listed for liver transplantation: An evidence-based review of COVID-19 screening modalities prior to transplant. Clin Liv Dis 15(6): 247-250.
- Zhang H, Dai H, Xie X (2020) Solid organ transplantation during the COVID-19 pandemic. Front Immunol. 11(1392): 1-9.
- Boyarsky BJ, Massie AB, Love AD, Werbel WA, Durand CM et al. (2020) Early experiences with COVID-19 testing in transplantation. Transplant Direct 6(7): e572.
- Han W, Zhu M, Zhang J, Zhu S, Li T, et al. (2020) Lung transplantation for elderly patients with end-stage COVID-19 pneumonia. Ann Surg 272(1): e33-e34.
- Pahari H, Shellagi N, Nath B (2020) Deceased donor liver transplantation in India in the COVID-19 era: Current scenario and future perspectives. Transplant Proc 52(9): 2684-2687.
- Sahin TT, Akbulut S, Yilmaz S (2020) COVID-19 pandemic: Its impact on liver disease and liver transplantation. World J Gastroenterol 26(22): 2987-2999.
- COVID-19 pandemic in Egypt.
- El-Meteini M, Fayez A, Abdalaal A, Safaan H, Mostafa I, et al. (2003) Living related liver transplantation in Egypt: an emerging program. Transplant Proc 35: 2783-2786.
- El-Meteini M, Hamza A, Abdalaal A, Fathy M, Bahaa M, et al. (2010) Biliary complications including single-center mortality: experience of 207 adult-to-adult living donor liver transplantations with right liver grafts. HPB 12(2): 109-114.
- Mouzaki M, Avinashi V, Babu A, Roestel KV, Deangelis M, et al. (2013) Basiliximab with delayed introduction of calcineurin inhibitors as a renal-sparing protocol following liver transplantation in children with renal impairment. Pediatr Transplant 17: 751-756.
- Lin CC, Chuang FR, Lee CH, Wang CC, Chen YS, et al. (2005) The renal-sparing efficacy of basiliximab in adult living donor liver transplantation. Liver Transplant 11(10): 1258-1264.
- Prem K, Liu Y, Russell TW, Kucharski AJ, Eggo RM, et al. (2020) The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health 5(5): e261-e270.
- Liu H, He X, Wang Y, Zhou S, Zhang D, et al. (2020) Management of COVID-19 in patients after liver transplantation: Beijing working party for liver transplantation. Hepatol Int 14(4): 432-436.
- Suissa S (1995) The case-time-control design. Epidemiol 6(3): 248-253.
- Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2): 205-213.
- Kumar D (2020) C4 article: Implications of COVID-19 in transplant. Am J Transplant.
- Escobar LE, Molina-Cruz A, Barillas-Mury C (2020) BCG vaccine protection from severe corona virus disease 2019 (COVID-19). Proc Natl Acad Sci USA 117(30): 17720-17726.
- (2021) Pfizer COVID-19 vaccine faces last step before FDA decision - Los Angeles Times. Accessed.
- Lamb Yvette N (2020) Remdesivir: First approval. Drugs 80(13): 1355-1363.
- Piemonti L, Landoni G (2020) COVID-19 and islet transplantation: Different twins. Am J Transplant. 20: 2983-2988.






























