Impact of Artifical Intelligence in Colonoscopic Polyp Detection and Classification
Leif Schiffmann1,2* and Ulrike W Denzer3
1 Department of General, Thoracic, Vascular and Transplantation Surgery, University of Rostock, Germany
2 Department of General, Visceral and Thoracic Surgery, Helios Klinikum Aue, Germany
3 Department of Gastroenterology, University Hospital Marburg, Germany
Submission:April 23, 2021; Published: April 30, 2021
*Corresponding author: Leif Schiffmann, Department of General, Thoracic, Vascular and Transplantation Surgery, University of Rostock, Schillingallee 35, 18057 Rostock, Germany
How to cite this article: Schiffmann L and Denzer UW. Impact of Artifical Intelligence in Colonoscopic Polyp Detection and Classification. Adv Res Gastroentero Hepatol, 2021; 16(5): 555948. DOI: 10.19080/ARGH.2023.16.555948.
Abstract
Background: Despite screening colonoscopy the relative risk for incidence and mortality of colorectal cancer accounts still for 50 to 60% probably due to missed adenomas. Artificial intelligence is a new development to rise detection of colorectal lesions and additionally to classify them.
Methods: We performed a Pubmed research for AI in combination with ADR, PDR and characterization of colorectal lesions.
Results: AI based detection of colorectal lesions rises ADR and PDR significantly, additionally withdrawal time is controlled. Standardized classification of bowel cleansing might be helpful. Nevertheless, rise of ADR and PDR is mainly based on the detection of small lesion with questionable relevance for colorectal cancer development within the control interval. Real-time characterization of detected colorectal lesions is currently on the level of expert endoscopist.
Conclusion: AI based colorectal polyp detection improves quality of screening colonoscopy in 2021 especially in not so experienced endocopists. Long time studies have to investigate influence on relevant outcome quality parameters especially incidence of colorectal cancer. AI based polyp characterization has currently to be improved before a leave in strategy of small benign lesions can be discussed
Keywords: Colorectal cancer; Artificial intelligence; Adenoma detection rate; Polyp detection rate; Polyp classification; Polyp characterization
Introduction
Colorectal cancer remains one of the most common of human malignancies and is responsible for 9% of deaths of malignancies worldwide [1]. Screening colonoscopy is a powerful tool preventing colorectal cancer [2]. With the widespread use of screening colonoscopy one could expect the rate of colorectal cancer dropping close to zero, but the relative risk for incidence and mortality is lowered only by around 50 to 60% [2]. Besides screening percentage and adherence to recommended screening intervals another discussed explanation are interval cancers based on missed colorectal lesions. There are several parameter which influence the adenoma and polyp detection rate including bowel preparation, time for withdrawal, second observer in unexperienced examiners, vigilance of the endocopist, endoscopy technique including high resolution imaging, virtual chromoendoscopy and tools for detection of lesions hidden behind mucosal folds and bends [3]. All these technical improvements lead to an increase of the ADR / PDR with the limitation of visualizing blind spots behind folds and curves. By reducing the latter, it appears almost impossible for a single endoscopist to analyze all the information in real-time.
Artificial intelligence might help to improve polyp detection automatically reducing blind spots and additionally classify these lesions. The aim of this review is to evaluate the current impact of artificial intelligence in polyp detection and characterization during the screening colonoscopy.
Methods
To evaluate the current impact of artificial intelligence in colonoscopy a literature research in Pubmed was undertaken searching for the terms “artificial intelligence” and “colonoscopy”, “polyp detection”, “adenoma detection”, “polyp classification” and “colorectal cancer”. All reviews were excluded. Out of this studies we selected studies considered valuable to be included in this work.
Results
AI and bowel preparation
The Boston Bowel Preparation score is mainly used for assessment of quality of bowel preparation. In case of insufficient bowel cleansing repetition of preparation and coloncoscopy is recommended due to current guidelines [4]. Computer based documentation and classification facilitates the assessment of bowel cleansing [5]. Zhou et al. developed ENDOANGEL – a deep convolutional neural network for the assessment of bowel preparation quality. The overall accuracy was 93.3%.
AI and withdrawal time
An adequate withdrawal time leads to careful inspection and detection of a higher number polyps and adenomas. Recommended minimal time is at least 6 minutes [6]. But, there are differences between the first four colonoscopies of a day`s work and all colonoscopies extending the number of nine. A study investigated that the latter are faster than the first with a decreasing PDR and ADR [7]. Artificial Intelligence might help to maintain the same accuracy for every colonoscopy no matter when it is scheduled in the day [8]. The ENDOANGEL system was tested to control withdrawal time as well as avoiding blind spots caused by slipping of the endoscope. The withdrawal time was significantly higher using the system (6.38 vs. 4.76 min) and the adenoma detection rate doubled (16 vs 8%) [5].
AI and detection of colonic lesions
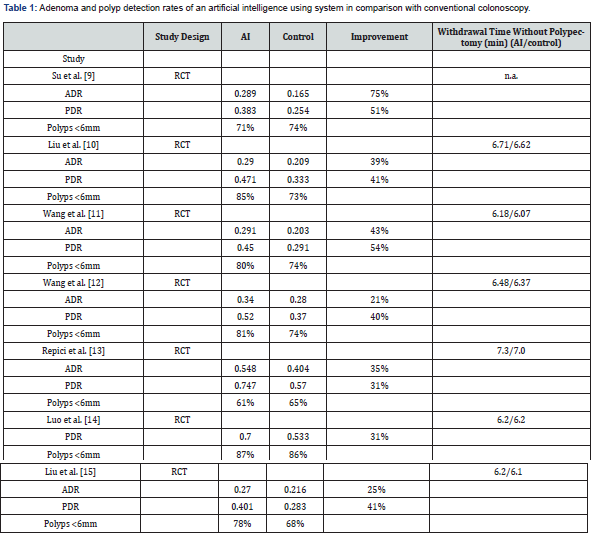
The main focus of AI is the computer based detection of colonic lesions resulting in an improvement of PDR and ADR. There are a few deep learning systems already commercially available that improve both by 21 to 75 percent for the ADR and 31 to 57 percent for the PDR (Table 1). Nevertheless the increase in the detection rate is based mainly on small lesions of 6 mm and below, the ones behind the folds, the flat lesions and the ones on the outer fields of sight [9-15]. Bigger lesions are diagnosed by either AI or an experienced endoscopist as well. Currently the impact of AI on the rate of interval carcinoma as a crucial clinical end point is not yet investigated.
AI and characterization of colorectal lesions
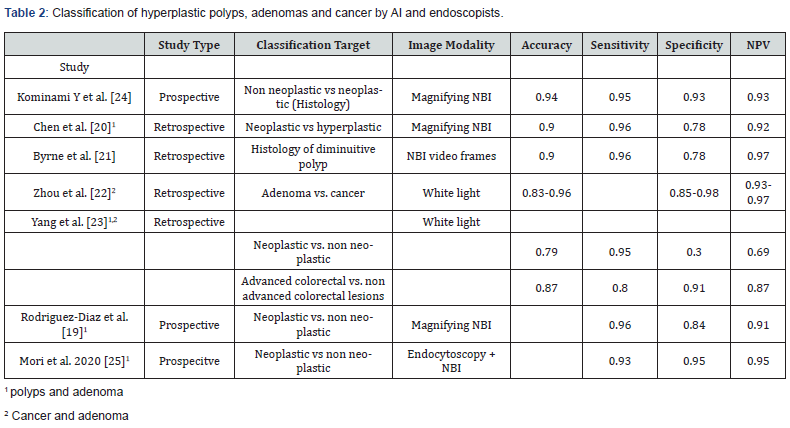
Especially in unexperienced endoscopists a computer aided diagnosis of polyp histology distinguishing between a hyperplastic polyp, non-neoplastic adenoma and a neoplastic adenoma would be helpful. Colorectal polyps are currently classified due to lesion morphology (Par’s classification) [16] and using image enhanced endoscopy and virtual chromoendoscopy due to surface and vessel pattern structure (e.g. Nice and JNET classification) for discrimination of hyperplastic and adenomatous lesions [17,18]. Structure and vessel changes allow a differentiation of non-neoplastic and neoplastic adenomas. Several studies investigated AI for characterization of colorectal polyps based on white light images, virtual chromoendoscopy with or without magnifying and pit pattern and vascularization features, table 2 summarizes the recent studies. The studies are heterogeneous concerning the image modalities used and their classification targets. Therefore, the results are differing with an sensitivity, specificity and negative predictive value ranging from 80 to 96%, 30 to 95% and 69 to 97% [19-23]. AI seems to be equivalent to expert endoscopist and appears to be advanced to beginners.
Discussion
Guidelines recommend the repetition of screening colonoscopy if the bowel cleaning is insufficient e.g. in case of a simplified BBS score ≤ 1 which means a bad preparation with lots of stool remaining. A computer based grading of bowel preparation may be more standardized especially for the in between stages than the individual endoscopists assessment which depends on experience. The AI system Endoangel [5] was evaluated based on the assessment of 5 experienced endoscopists after a special training and the scoring was taken into account only if 3 of the 5 experts were congruent. More than 5000 images were labeled to the BBS score. The system might be helpful to compare bowel preparation quality in a more objective manner and to evaluate the influence of bowel preparation to the ADR and PDR in intermediate phases of bowel cleansing in future studies.
The influence of withdrawal time and ADR was already proved in 2006 [6] to be at least 6 minutes. Blind spots caused by slipping during a too rapid withdrawal are likely to decrease ADR/ PDR [8]. Studies investigating AI showed a higher rate of detected polyps withdrawal time may shorten during a day’s course and the more subjective sense of time with a higher stress level of the endoscopist. The study of Gong et al. using the Endoangel system demonstrated a decline of withdrawal time below 6 minutes in the control group [8]. AI might be helpful to find blind spots and raise the ARD but is it really necessary for meeting the mandatory withdrawal time? Nevertheless, if using an AI system control of withdrawal time should be included. If not using AI it seems to be recommendable to be aware of someone’s own withdrawal times during the day.
AI based detection of colorectal polyps assists the endoscopist to rise the PDR and the ADR. This is helpful – especially for beginners. Less supervision is necessary and resources are saved. Wieszczy et al. showed, that an ADR below 20% results in an at least doubling risk of developing a manifest colorectal cancer within 10 years, even if no adenomas were detected in the initial colonoscopy [15]. This underlines the importance of carefully inspection during screening colonoscopy. Studies investigating AI in colorectal polyp detection (Table 1) proved a higher rate of detected polyps, albeit the majority of the higher output were small benign lesions below 6 mm with questionable relevance for cancer formation within the recommended control intervals. Clinical input of the AI systems concerning long time outcome quality e.g. lowering carcinoma incidence with screening colonoscopy is still unclear and has to be investigated in long time studies.
Future perspective would be a precise AI characterization of hyperplastic polyps, non-neoplastic and neoplastic adenoma, in the latter group preferentially distinguishing between early cancer and submucosal invading cancer. This would allow to consider a resect and discart strategy during removing of benign lesions. Moreover one could discuss about leaving small hyperplastic polyps in place [20]. Currently the data of AI based characterization of colorectal polyps are still of variable quality. Studies are using different image modalities and different classification targets and size of datasets. Negative predictive values range from 0.69 to 0.97. With further development of AI one can expect a rising differentiation quality of the systems enabling us to a virtual biopsy of e.g. hyperplastic polyps. Up to that we will continue to polyp resection and histologic work up.
Conclusion
In conclusion AI based colorectal polyp detection improves quality of screening colonoscopy in 2021 especially in not so experienced endocopists. Standardized grading of bowel preparation and control of withdrawal time seem to be helpful. Long time studies have to investigate influence on relevant outcome quality parameters especially incidence of colorectal cancer. AI based polyp characterization has currently to be improved before a leave in strategy of small benign lesions can be discussed.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 68(6): 394-424.
- Zhang J, Chen G, Li Z, Zhang P, Li X, et al. (2020) Colonoscopic screening is associated with reduced Colorectal Cancer incidence and mortality: a systematic review and meta-analysis. J Cancer 11(20): 5953-5970.
- Allescher HD, Weingart V (2019) Optimizing Screening Colonoscopy: Strategies and Alternatives. Visc Med 35(4): 215-225.
- Hassan C, Bretthauer M, Kaminski MF, Polkowski M, Rembacken B, et al. (2013) Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 45(2): 142-150.
- Zhou J, Wu L, Wan X, Shen L, Liu J, et al. (2020) A novel artificial intelligence system for the assessment of bowel preparation. Gastrointestinal Endoscopy 91(2): 428-435.
- Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL (2006) Colonoscopic Withdrawal Times and Adenoma Detection during Screening Colonoscopy. N Engl J Med 355(24): 2533-2541.
- Marcondes FO, Gourevitch RA, Schoen RE, Crockett AD, Morris M, et al. (2018) Adenoma Detection Rate Falls at the End of the Day in a Large Multi-Site Sample. Dig Dis Sci 63(4): 856-859.
- Gong D, Wu L, Zhang J, Mu G, Shen L, et al. (2020) Detection of colorectal adenomas with real-time computer-aided system (ENDOANGEL): a randomized controlled study. Lancet Gastroenterol Hepatol 5: 352-361.
- Su JR, Li Z, Shao XJ, Ji CR, Ji R, et al. (2020) Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: a prospective randomized controlled study. Gastrointestinal endoscopy 91(2): 415-424.
- Liu P, Wang P, Brown JRG, Berzin TM, Zhou G, et al. (2020) The single monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study. Ther Adv Gastroenterol 13: 1-13.
- Wang P, Berzin TM, Brown JRG, Bharadwaj S, Becq A, et al. (2019) Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomized controlled study. GUT 68(10): 1813-1819.
- Wang P, Liu X, Berzin TM, Brown JRG, Liu P, et al. (2020) Effect of a deep-learning computer aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomized study. Lancet Gastroenterol Hepatol 5(4): 343-351.
- Repici A, Badalamenti M, Maselli R, Correale L, Radaelli F, et al. (2020) Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 159(2): 512-520.
- Luo Y, Zhang Y, Liu M, Lai Y, Liu P, et al. (2020) Artificial Intelligence-Assisted Colonoscopy for Detection of Colon Polyps: a Prospective, Randomized Cohort Study. J Gastrointestinal Surg.
- Wieszczy P, Waldmann E, Loberg M, Regula J, Rupinski M, et al. (2020) Colonoscopist Performance and Colorectal Cancer Risk After Adenoma Removal to Stratify Surveillance: Two Nationwide Observational Studies. Gastroenterology 160(4): 1067-1074.
- Lambert R, Kudo SE, Vieth M, Allen JI, Fujii H, et al. (2009) Pragmatic classification of superficial neoplastic colorectal lesions. Gastrointest Endosc 70(6): 1182-1199.
- Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, et al. (2012) Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 143(3): 599-607e.
- No Y, Tanaka S, Kudo SE, Saito S, Matsuda T, et al. (2016) Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc 28(5): 526-533.
- Rodriguez-Diaz E, Baffy G, Lo WK, Mashimo H, Vidyarthi G, et al. (2020) Real-time artificial intelligence-based histological classification of colorectal polyps with augmented visualization. Gastrointestinal Endoscopy 93(3): 662-670.
- Chen PJ, Lin MC, Lai MJ, Lin JC, Lu HHS, et al. (2018) Accurate Classification of Diminutive Colorectal Polyps using Computer-Aided Analysis. Gastroenterology 154(3): 568-575.
- Byrne MF, Chapados N, Soudan F, Oertel C, Pérez ML, et al. (2019) Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut 68: 94-100.
- Zhou D, Tian F, Tian X, Sun L, Huang X (2020) Diagnostic evaluation of a deep learning model for optical diagnosis of colorectal cancer. Nature Communications.
- Yang JY, Cho BJ, Lee MJ, Kim JH, Lim H, et al. (2020) Automated Classification of Colorectal Neoplasms in White-Light Colonoscopy Images via Deep Learning. J Cin Med 9: 1593.
- Kominami Y, Yoshida S, Tanaka S, Sanomura Y, Hirakawa T, et al. (2016) Computer-aided diagnosis of colorectal polyp histology by using a real-time image recognition system and narrow-band imaging magnifying colonoscopy. Gastrointest Endosc 83: 643-649.
- Mori Y, Kudo S, East JE, Rastogi A, Bretthauer M, et al. (2020) Cost savings in colonoscopy with artificial intelligence-aided polyp diagnosis: an add-on analysis of a clinical trial. Gastrointestinal Endoscopy 92(4): 905-911.






























