Selected Highlights from the Recent Literature of Newly Reported Herbal and Dietary Supplement-Induced Liver Injury
Jessica J Rosenberg1 Cory Higley1 and James H Lewis2
1 Department of Internal Medicine, MedStar Georgetown University Hospital, USA
2Department of Gastroenterology, MedStar Georgetown University Hospital, USA
Submission:April 28, 2020; Published:May 11, 2020
*Corresponding author:Jessica Rosenberg, Georgetown University Pasquerilla Healthcare Center, 6th Floor 3800 Reservoir Road, N.W. Washington, DC 20007, USA
How to cite this article:Jessica J R Cory H, James H L. Selected Highlights from the Recent Literature of Newly Reported Herbal and Dietary Supplement-Induced Liver Injury. Adv Res Gastroentero Hepatol, 2020;15(1): 555904. DOI: 10.19080/ARGH.2020.15.555904.
Abstract
Herbal and dietary supplements (HDS) encompass a large number of products with a wide range of ingredients. The use of HDS and the risk of hepatotoxicity is well documented; however, despite increasing awareness and many publicized cases, instances of new HDS-related injury continue to occur. Here, we highlight recent case reports, reviews, and research describing relatively new herbal and dietary supplement-induced liver injury. In particular, we focus on several of the more recently implicated products, such as kratom, weight loss supplements including multi-ingredient products (such as OxyELITE-Pro), Garcinia cambogia, CBD oil and energy drinks.
Keywords: Herb-induced liver toxicity; Herbal and dietary supplements; Hepatotoxicity; Drug-induced liver injury; Kratom; Cannabidiol; Weight loss supplements; Energy drinks/p>
Abbreviations: HILI: Herb-Induced Liver Injury; DILIN: US Drug‐Induced Liver Injury Network; RUCAM: Roussel Uclaf Causality Assessment Method; HDS: Herbal and Dietary Supplements; DSHEA: Dietary Supplement Health and Education Act
Introduction
The term herbal and dietary supplements (HDS) describes a wide range of ingredients, including vitamins, minerals, proteins, and herbal agents used for general dietary supplementation or for specific goals such as gaining muscle mass, increasing energy, or weight loss. The types of herbal products and the frequency with which they are used varies significantly by global region. In parts of Asia, for example, traditional Chinese medicines including herbs and dietary supplements are ubiquitous and integrated into the health care system. In contrast, in many Western countries, HDS are considered to be complementary or alternative medicines (CAM) and often lack the same oversight and regulatory requirements that are required for prescription pharmaceuticals. As a result, the ingredients as well as the safety and efficacy claims of HDS may be less reliable. An estimated 40.6 million adults in the United States reported using herbal and dietary supplements in 2012 [1] and the industry now exceeds $40 billion annually in the U.S [2].
The risk of hepatotoxicity from various HDS products is well known [3-8]. The Drug Induced Liver Injury Network (DILIN) has reported that herbal supplement attributed liver injury ranges from 2% to 16% of all reported hepatotoxicity in the United States [5] and has more than doubled in the past decade. The proportion of drug-induced liver injury (DILI) attributed to herbal products is much higher in Asian countries [9-12].
Despite increasing awareness and many highly publicized cases, instances of HDS-related injury continue to occur. Our knowledge base on specific agents, incidence, and risk factors for herbal-induced liver injury (HILI) continues to grow worldwide. Herein, we provide highlights of recent case reports, case series, reviews, and research in the field of herbal and dietary supplement-induced liver injury. In particular, we focus on several of the more recently described HDS agents, including kratom, Garcinia gambogia, weight loss supplements, multi-ingredient products (such AS OxyELITE Pro), CBD oil, and energy drinks, as well as discussing some of the more controversial aspects, such as regulation of HDS.
Methods
We conducted a literature review using the PubMed search engine, specifically searching for the terms such as “herbal”, “hepatotoxicity”, and “liver injury”. We limited our results to articles published in English and, with rare exception, excluded animal or in vitro studies. Our search was limited to articles published and indexed to PubMed between 2018 and March 31, 2020. Older reports were also reviewed for proper context of these newer publications. We reviewed case reports, case series, review articles, meta-analyses, and original research in addition to updates from national and international drug-induced liver injury (DILI) registries. From these, we chose to include those publications we thought would hold the most interest to readers interested in recently implicated agents causing HILI and HDS hepatotoxicity, emphasizing what we felt were the most informative for clinical practice.
Epidemiology and Risk Factors
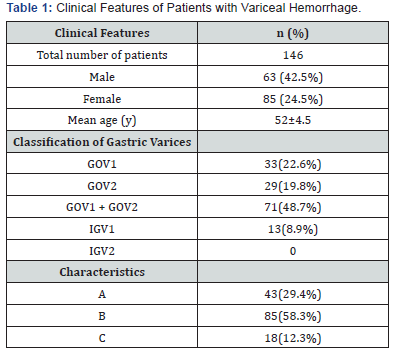
The total impact of HDS-associated liver injury worldwide is unknown as causality is difficult to establish and the true prevalence of HDS use is not known. In contrast to drug-induced liver injury (DILI), there have not been any large populationbased studies allowing for the assessment of incidence of HILI in the United States. The U.S. Drug Induced Liver Injury Network (DILIN), however, has reported that herbal products are associated with between 2% to 16% of all reported hepatotoxicity, and the incidence has nearly tripled in the last decade, rising from 7% to 20% over that time [5]. Of course, not all herbal agents are hepatotoxic. For example, in the LiverTox database compiled by Hoofnagel and colleagues from the Liver Disease branch at the NIH and the National Library of Medicine [13], more than 60 HDS agents are currently listed, although nearly 40% have no evidence to implicate them as hepatotoxins. Similarly, although more than 50 traditional Chinese medicines (TCM) have been associated with hepatic injury, causality has been established for only about half of these compounds [14] (Table 1).
Andrade et al. [15] recently published a summary of available data on liver injury from herbal and dietary supplements in Europe, Latin America, and Asia. Of note, the relative prevalence of HDS liver injury from traditional medicines and dietary supplements appears to be notably different across the Asian countries, with 17.1% in Japan, 18.6% in China, 71% in Singapore, and 72.7% in Korea. These percentages parallel the findings from other case series and registries from Singapore [9], China [11,16] and Korea [12]. Similar to the US DILIN findings, the Spanish DILI Registry also found the yearly proportion of HILI has increased substantially, going from 1.5% in 1998 to 6% of the cases identified from 2014 to 2016 [6]. Herbal and dietary supplements products were responsible for 4% (32 cases) of the 856 DILI cases in the Spanish DILI Registry. The HILI group showed a particular pattern: a female predominance (63%), a hepatocellular injury pattern (94%), and a greater severity evolving more frequently into acute liver failure (6%) than conventional DILI (4%).
A systematic review and case control study by Lin et al. [7] examined HILI risk factors using data from 53 articles published worldwide. These authors found that the most commonly reported herbal supplements are greater celandine (22.6%), germander (22.6%), and chaparral (9.4%). Overall, HILI was again more common in women (69.8 %), although among the elderly, men were found to be more commonly affected (37.5% vs 10.5%, P = .02)
Mislabeling/Misbranding of HDS
The presence of unlabeled ingredients is of increasing concern among herbal and dietary supplements. To further investigate the magnitude of this risk, Navarro et al, [21] studied the HDS collected from DILIN enrollees to determine the accuracy of product labels and to identify potential hepatotoxins. They found that among 341 HDS agents from 1,268 patients with suspected DILI, only 272 of these products had labels listing their ingredients. These compounds then underwent sophisticated chemical analysis using ultrahigh‐performance liquid chromatography coupled with mass spectrometry at the National Center for Natural Products Research at the University of Mississippi in Oxford, MS. Of the 272 products tested, only 132 had labels that accurately reflected the compounds identified by chemical analysis; therefore, 140 (51%) were mislabeled. When categorized by their marketed indications, the rates of mislabeling were highest for those enhancing sexual performance, appearance, energy boosters, and weight loss products. Importantly, several products contained unlabeled adulterants, including known hepatotoxins such anabolic steroids, diclofenac, and tamoxifen. These findings suggest the need for more stringent regulatory oversight to verify product contents and labeling in an effort to reduce potential hepatotoxicity.
Weight loss supplements
Dietary supplements promoted for weight loss encompass a wide variety of products with varying mechanisms of action. Approximately 15% of U.S. adults have used a weight-loss dietary supplement at some point in their lives, with more women reporting their use (21%) than men (10%) [22].
OxyELITE Pro is the commercial name for a variety of multiingredient nutritional supplements marketed for weight loss and body building. Various formulations of this supplement have been implicated as a cause of liver injury since 2012 when FDA had received more than 100 adverse drug reports from 33 states, Puerto Rico and a number of foreign countries dating to 2010, with nearly 50 instances of clinically apparent acute liver injury known [23]. In 2013 the FDA banned the use of the original active ingredient, 1,3-dimethylamyylamine in all nutritional supplements [23], a stimulant linked to hypertension, heart attacks, seizures, psychiatric disorders and death [24]. OxyELITE Pro was reformulated using aegeline as the active ingredient, an alkaloid extract from the leaves of the Asian bael tree (Agele marmelos), but following initial reports Hawaii and the military, and dozens of additional adverse reports [25-28] and the FDA issued a warning to avoid the reformulated product in October 2013 and the manufacturer was required to cease production and destroy all remaining retail product [28,29]. Subsequent animal studies of the new formulation also documented its hepatotoxic potential [31]. Despite its supposed removal, new cases have appeared. Fontana et al. [32] recently described a case of severe acute hepatitis attributed to this herbal and dietary supplement. A 31‐year‐old Korean American female presented with new‐onset nausea, vomiting, and pruritus after taking one tablet of OEP per day for the past 4 months. Her initial serum AST was 710 IU/L, ALT 1972 U/L, alkaline phosphatase was 58 U/L, total bilirubin was 3.8 mg/dL, and INR was 1.0. The DILIN causality score was 1 (definite), and her Roussel Uclaf Causality Assessment Method (RUCAM) score was 7 (probable). Although aegeline has been used in Ayurvedic medicine for centuries; the mechanism by which aegeline and/or other components of OxyELITE Pro resulted in liver injury remains unknown.
Another report illustrates the significant morbidity associated with these “fat burning” (or thermogenic) supplements, in this case, Thermo Gun. A 36-year-old male patient developed jaundice 7 days after he started taking this thermogenic agent, and he subsequently developed acute liver failure requiring liver transplantation [33]. The supplement was found to contain N-acetyl-L-tyrosine, 1,3,7-trimenthylxanthine, white willow, and 1-hydroxypholedrine, although no prior reports of acute liver failure were found in the literature associated with these particular substances. The RUCAM of 7 was calculated, indicating a probable association between use of this supplement and liver injury.
Hydroxycut, a multi-ingredient nutritional supplement marketed for weight loss, fat-burning and body building is well-known to be linked to hepatotoxicity. The FDA issued a warning against the use of Hydroxycut products in 2009 and recalled it from the market based on nearly two dozen reports of possible hepatotoxicity, including 2 patients who required liver transplantation [34,35]. The specific ingredient responsible for the acute liver injury has not been identified, although ephedra (Ma huang) and Camellia sinensis from green tea extracts have been implicated. Currently, however, several reformulated Hydroxycut products remain on the market, and a recently reported case points to its ongoing hepatotoxic potential [36]. Khetpal et al, [35] described a 22-year-old obese woman who had been taking these products for about 3 months and presented with marked elevations of ALT 2399 U/L and AST 4040 U/L with normal alkaline phosphatase 72 U/L and total bilirubin 0.6 mg/dl. Her INR was mildly prolonged at 1.4. A RUCAM score of 9 suggested it was associated with a high possibility of DILI. She was hospitalized and treated with N-acetylcysteine. The aminotransferases trended downward during the course of her hospitalization by day 8.
Garcinia cambogia
Garcinia cambogia (GC) is an herbal supplement that has only relatively recently been associated with liver injury. Frequently used as a weight loss supplement, this tropical fruit with the active ingredient hydroxycitric acid is thought to act by decreasing de novo synthesis of fatty acids and glycogen storage via inhibition of ATP citrate lyase, while decreasing appetite. Its use has been linked to cases of acute hepatitis, including instances of acute liver failure requiring transplantation [37-40].
A 2018 study in Italy described four cases of hepatotoxicity in women aged 39 to 61 years taking GC extract for weight loss, from data collected by the Italian Surveillance System of Natural Health Products coordinated by the Italian National Institute of Health [37]. Causality scores from the Council for International Organization of Medical Sciences (CIOMS) were calculated to be 6 or 7 for each case, suggestive of a probable diagnosis of HILI. The authors also performed a literature review and identified 24 additional case reports and 8 case series documenting adverse events in a total of 66 patients after consumption of GC. Of these 32 reports, 17 had documented hepatotoxicity, including acute liver failure, in a total of 50 patients. Causality scores were calculated in nearly all instances, using CIOMS, WHO, and DILIN criteria, and all were graded as possible, probable, highly likely and in some cases, certain.
Additional cases of hepatotoxicity linked to GC continue to appear. Sharma et al, [41] reported an acute case of hepatitis from GC in a 57-year-old woman which resolved after stopping the supplement and recurring after she resumed taking it (constituting a positive rechallenge response). The supplement was noted to be 100% pure GC fruit rind extract, in a dose of 2800 mg daily. Once she initially discontinued the supplement, her liver enzymes decreased significantly with normalization of INR and PT. However, 6 months later, the patient was again found to have an elevated ALT of 301 and AST of 69 and it was found that she had started taking the same supplement again. The CIOMS/ RUCAM score was calculated to be 11 for the patient, indicating causality was highly probable.
Yousaf et al, [42] reported a case of 21-year-old morbidly obese female who presented with abdominal pain for 1 week associated with nausea, vomiting, anorexia, and myalgias, who was found to have been using a weight loss herbal supplement containing Garcinia cambogia for 4 weeks. Laboratory workup revealed elevated ALT 981 U/L, AST 1062 U/L, alkaline phosphate 248 U/L, and international normalized ratio (INR) 1.6. In this patient, a RUCAM score of 9 indicating a probable association with GC. Her liver tests returned to her baseline after 42 days following discontinuation of the product.
These case reports continue to reinforce the potential toxic effects of GC contributing to hepatotoxicity. More research is needed to clarify the association between GC, hydrocitric acid, and hepatotoxicity and whether this herbal agent needs to be more closely regulated, given its association with serious and potentially fatal hepatic complications.
Tea-induced liver injury
Several cases of HILI associated with tea have been recently published. Rodriquez et al [43] described the first case of a man with acute hepatitis secondary to the use of Ilex paraguariensis, also known as yerba mate, an herbal product commonly drunk as a tea in South America. Green tea extract (GTE), is derived from the Chinese tea tree Camellia sinensis, with the active ingredient thought to be epigallocatechin gallate (EGCG) The presence of catechins containing GTE have been implicated in instances of hepatotoxicity [44]. A previous randomized controlled trial [45] demonstrated the association of GTE with a rise in liver enzymes and a recent report describes severe hepatitis potentially related to the consumption of green tea in a 2-year-old child [46]. As part of the United States Pharmacopeia’s (USP) ongoing review of dietary supplement safety data, a new systematic review summarized the evidence on the safety of GTE, including toxicological studies that show a hepatocellular pattern of liver injury, with hepatotoxicity resulting from 140 mg to 1000 mg/day of EGCG. Although there was substantial inter-individual variability in susceptibility, possibly due to genetic factors, based on these findings the USP has included a cautionary labeling requirement in its Powdered Decaffeinated Green Tea Extract monograph warning against signs and symptoms of liver injury [47].
Bodybuilding Agents
Bodybuilding supplements, used for the purposes of performance enhancement and muscle building, are generally comprised of anabolic androgenic steroids (AAS). These supplements are associated with liver abnormalities including severe and prolonged cholestatic liver injury, with the suspected hepatotoxic agent being C-17 alkylated testosterones. The U.S. Drug‐induced Liver Injury Network (DILIN) prospective study found that 8% of HILI cases in 2010 to 2012 were due to bodybuilding supplements, and these products were the most common cause for liver injury in those using HDS products. [5] Recently, Stolz et al [48] sought to further describe the US DILIN’s experience with body building supplements. They summarized the clinical, laboratory, and histological features of 44 patients with liver injury due to bodybuilding supplements. The authors found that patients with liver injury due to bodybuilding supplements presented with a uniform and distinctive pattern of marked cholestatic liver injury with jaundice and pruritus, that was often prolonged and accompanied by disability and weight loss. However, when compared to non-body building HDS products in which liver injury occurred predominantly middleaged women and were hepatocellular in nature, the bodybuilding products linked to hepatotoxicity were more often reversible and less likely to lead to death or transplant (1% vs 13%).
Kratom
Kratom (Mitragyna speciosa) is a plant indigenous to parts of Southeast Asia, where its leaves have long been used to treat common health conditions, alleviate pain, and as use as a stimulant. More recently, however, Kratom is being increasingly used in the United States for the self-management of opioid withdrawal and treatment of pain [49]. The CDC has estimated a >10-fold increase in kratom use linked to opioid overdoses between 2010 and 2015 [50] and recent cross-sectional study estimated a lifetime use among adults of 1.3%, representing 3,353,624 adults [51]. Its active components are mitragynine and 7 hydroxymitragynine, which act as partial mu and delta opioid receptor agonists, with the former mediating euphoria, analgesia and respiratory depression [52]. Currently, kratom is marketed and regulated as a food and/or dietary ingredient. However, the hepatotoxic effects of kratom continue to be documented in the literature [53,54]. A recent comprehensive review of human cases [55] found that latency periods to symptom onset had a median of 20.6 days and mean of 21 days (range 2-49 days). Common presenting signs and symptoms were abdominal discomfort, jaundice, pruritus, and dark urine, and histologic findings have been predominantly acute zone 3 cholestasis with mild portal inflammation. In some cases, bile duct injury mimicks that seen with PBC, although antimitochondrial antibodies are absent [56,57].
Limited basic science and clinical research studies suggest that kratom may have beneficial therapeutic properties; however, no randomized-controlled clinical trials have been performed to determine the true risks and benefits of kratom use [58]. There have been reports of other serious adverse effects including seizures and death among kratom users. Given these concerns, the US Drug Enforcement Administration (DEA) announced plans to place the main active constituent of kratom in Schedule I of the Controlled Substances Act. This action sparked public debate and protest, and interestingly, several months later, the DEA withdrew its notice of intent to schedule kratom due to widespread backlash [59]. The FDA has cited kratom as an opioid, citing its potential for abuse and addiction, prompting caution from others [60,61]. A congressional committee has recently expressed concerns about barriers to research created by a potential Schedule I status, requesting that NIH expand research on kratom including its constituent compounds recommending an additional $3 million in appropriations to tackle such research [62]. Such policy may allow for important research which is needed to understand the potential efficacy and safety of kratom as well as its addictive potential.
Energy Drinks
Energy drink consumption in the United States has increased substantially over the past decade among adolescents, young adults, and middle-aged adults [63]. Energy drinks are nonalcoholic beverages that contain caffeine or other plant-based stimulants, amino acids (e.g., taurine), herbs (e.g., ginkgo biloba), and vitamins. While most of the reported adverse effects relate to the cardiovascular and neurological systems, there are a growing number of reports linking energy drinks to liver injury. We have identified a small number of case reports and summarized these six case reports below in Table 2.
All cases involved a relatively young adult population, aged 16 to 50, and all presented after consuming an excessive quantity of energy drinks. Infectious, autoimmune, and known hepatotoxins were ruled out as etiologies in all cases. Notably, one patient eventually developed acute liver failure and required transplantation [66]. The mechanism of liver injury from these energy drinks remains unclear. The ingredients of these products vary, but most of them contain caffeine, L-carnitine, taurine, B vitamins, glucuronolactone, antioxidants, trace minerals, guarana, sucrose, Ginkgo biloba, and/or ginseng [70]. In one case, the patient’s high levels of serum folate and vitamin B12 were thought to be consistent with supplemental intake from energy drink usage as the patient denied any alternative supplementation. The ingredient most often cited as being responsible for the development of HILI is vitamin B3 (niacin), although it is worth noting that the quantity of niacin consumed is often below the threshold expected to cause toxicity [71]. Importantly, the precise quantities of all the ingredients are not known – for example, there are several energy drinks that do not clearly label the amount of caffeine – and little is known about the toxicity profiles of some of the other compounds in these energy drinks or about the interactions between them.
The imprecise and inconsistent labeling is partly a function of how energy drinks are regulated. In the past, energy drinks were typically classified as dietary supplements and existed outside the purview of the U.S. Food and Drug Administration (FDA). Now, energy drink manufacturers can choose whether to label their products as a “beverage” or as a “dietary supplement”. Energy drinks classified as beverages must comply with the Nutrition Labeling and Education Act of 1990 (NLEA) that labels the drinks with conventional Nutrition Facts panels. In contrast, energy drinks designated as dietary supplements must comply with the labeling requirements of the Dietary Supplement Health and Education Act (DSHEA) of 1994, which are less stringent. In 2014, in response to the rising popularity and concern over the safety of energy drinks, the FDA issued a series of industry guidances “to help dietary supplement and beverage manufacturers determine whether a liquid food product is properly classified as a dietary supplement or as a beverage, and to remind the industry of legal requirements regarding the substances that may be added to either type of product [71].” It is important to note that FDA guidance documents do not establish legally enforceable responsibilities and are merely recommendations. As concern over the potential toxicity of energy drinks grows, it may be that additional consideration needs to be given to more stringent regulation of these products.
Cannabidiol (CBD Oil)
Cannabis-based products for medicinal use contain cannabinoids derived from the cannabis plant, including delta-9- tetrahydrocannabinol (THC), cannabidiol (CBD), or a combination of THC and CBD. CBD oil is a non-psychotropic derivative of cannabis sativa used for the management and treatment of number of medical conditions [72]. It is a major ingredient in Epidiolex, a medication introduced in 2018 for management of Dravet syndrome (DS) and Lennox-Gastaut Syndrome (LGS), conditions in which epilepsy can be refractory to traditional antiepileptic medications [73,74]. Increases in ALT >3X ULN (without any rise in serum bilirubin) were seen in 10-20% of patients in human trials, and a dose-response was appreciated [75]. Elevated aminotransferases usually resolved spontaneously after discontinuation or dose-reduction (or of valproic acid that was taken concomitantly), and there were no events of liver failure or death related to liver injury. Liver injury was noted most frequently in the first 30-90 days of treatment and was rare after 200 days of treatment. Therefore, it has been recommended that patients be monitored frequently for 3 months and then at regular intervals for 1 year of treatment and then at 6 to 12-month intervals thereafter. The FDA issued a press announcement in late November 2019 detailing safety concerns over CBD oil, stating there is insufficient evidence to conclude that CBD oil is safe, citing the potential for liver injury among other adverse effects [76]. A study addressing the short-term toxicity of a CBD-rich extract in a rodent model specifically designed to investigate the hepatotoxicity potential of CBD was recently published [77]. This study showed that CBD, when delivered orally to mice in the form of a concentrated CBD-enriched cannabis extract, has the potential to cause liver injury, with the highest CBD dose (2460 mg/kg) exhibiting clear evidence of hepatotoxicity as indicated by marked increases in serum ALT, AST, and total bilirubin as well as increased intrahepatic concentrations of oxidized glutathione. Although this dosing is not applicable to most real-life scenarios, it does provide information regarding the potential consequences of CBD overdose.
Given the emerging evidence suggesting that CBD has the potential to induce drug interactions [74], with CBD having been shown to modulate enzymes involved in drug metabolism and drug-induced hepatotoxicity (e.g., APAP and ethanol), a recent study was done to investigate the CBD/APAP interaction potential in mice [78]. This showed an in vivo potential for a CBD/ drug interaction resulting in liver injury, with mice treated with clinically relevant CBD doses followed by a challenge with APAP that resulting in the development of a sinusoidal obstruction syndrome (SOS)-like pattern of liver injury and mortality [78]. Such herb-drug interactions are the subject of ongoing study [79].
There are currently no other FDA-approved drug products that contain CBD. At present, according to the FDA, neither CBD nor THC are excluded from the dietary supplement definition and cannot be sold or marketed as such. However, CBD continues to be marketed in the US as a dietary supplement, with manufacturers making various health claims, such as its use for pain relief and managing depression [80].
Summary
HDS product use worldwide is substantial, with nearly 50% of adult Americans reporting use of an HDS product [81]. In the United States, there have been no large prospective cohort studies with which to estimate nation-wide incidence of HILI, although reports of hepatotoxicity have been increasing in the US and other westernized countries, and it is even higher in many Asian nations Causality remains difficult to establish for a host of reasons, including multi-ingredient supplements, co-ingestion with pharmaceutical medications, and the relatively low frequency with which HDS use is disclosed to health care providers [82].
While the general public may perceive HDS products as being safer than conventional medications, given that HDS are often derived from “natural” products such as plants, many have been linked to significant liver injury and remain widely available without a prescription over the internet and in so-called health food stores. It is of note that cases of hepatotoxicity are now being reported from herbal and dietary supplements that are commonly considered relatively benign, such as teas and energy drinks. CBD, one of the main components of cannabis, is approved by the FDA for use in only one product, a prescription drug used to treat two severe forms of epilepsy. While it is not legally considered a dietary supplement, much interest centers around CBD and its potential health benefits, and it has been marketed in the US as a dietary supplement for various health claims, such as relief of chronic pain and depression. Several randomized controlled trials have reported liver toxicity associated with CBD, and recent animal studies have demonstrated its hepatotoxic potential as well as its potential to induce drug interactions.
Although HDS-associated liver injury remains rare overall, its significance is often marked by more severe liver injury and worse outcomes compared with DILI from conventional drug use. The need remains for a better understanding of the incidence and risk factors of herbal and dietary supplement-associated liver toxicity, the presence of unlabeled ingredients, and to determine the causative hepatotoxic agents and their mechanism of liver injury. For example, a form of immune-mediated hepatotoxicity was recently described for Polygonal multiflorum, which serves as an example of newer potential mechanisms for other HDS as well [83]. As HDS products in the US require no specific evidence of safety or efficacy testing prior to marketing based on the Dietary Supplement Health and Education Act of 1994, calls are increasing being made for more rigorous oversight into their manufacturing and indication claims to improve the safety of these increasingly used products [84-86].
References
- Wu CH, Wang CC, Tsai MT, Huang WT, Kennedy J (2014) Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 national health interview surveys. Evid Based Complement Alternat Med 2014: 872320.
- Ostroff S (2017) Public meeting to discuss the development of a list of pre-DSHEA ingredients.
- Teschke R, Wolff A, Frenzel C, Schulze J (2014) Review article: herbal hepatotoxicity – an update on traditional Chinese medicine preparations. Aliment Pharmacol Ther 40(1): 32-50.
- Brown AC (2017) Liver toxicity related to herbs and dietary supplements: online table of case reports. Part 2 of 5 series. Food Chem Toxicol 107(part A): 472-501.
- Navarro V., Barnhart H., Bonkovsky HL, Davern T, Fontana RJ, et al. (2014) Liver injury from herbals and dietary supplements in the U.S. drug induced liver injury network. Hepatology 60(4): 1399-14086.
- Medina-Caliz I, Garcia-Cortes M, Gonzalez-Jimenez A, Cabello MR, Robles-Diaz M, et al. (2018) Herbal and dietary supplement-induced liver injuries in the Spanish DILI Registry. Clin Gastroenterol Hepatol 16(9): 1495-1502.
- Lin NH, Yang HW, Su YJ, Chang CW (2019) Herb induced liver injury after using herbal medicine: A systemic review and case-control study. Medicine (Baltimore) 98(13): e14992.
- Seeff LB, Bonkovsky H, Navarro V, Wang G, et al. (2015) Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology 148(3): 517-532.
- Patel DN, Low WL, Tan LL, Tan MM, Zhang Q, et al. (2012) Adverse events associated with the use of complementary medicine and health supplements: an analysis of reports in the Singapore Pharmacovigilance database from 1998 to 2009. Clin Toxicol (Phila) 50(6): 481-489.
- Yang LX, Liu CY, Zhang LL, Lai LL, Fang M, et al. (2017) Clinical characteristics of patients with drug-induced liver injury. Chin Med J (Engl) 130(20): 160-164.
- Shen T, Liu Y, Shang J, Xie Q, Li J, et al. (2019) Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology 156(8): 2230-2241.
- Lee J, Shin JS, Kim MR, Byun JH, Lee SY, et al. (2015) Liver enzyme abnormalities in taking traditional herbal medicine in Korea: a retrospective large sample cohort study of musculoskeletal disorder patients. J Ethnopharmacol 169: 407-412.
- (2012) Liver Tox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases.
- Teschke R, Zhang L, Long H, Schwarzenboeck A, Schmidt-Taenzer W, et al. (2015) Traditional Chinese Medicine and herbal hepatotoxicity: a tabular compilation of reported cases. Ann Hepatol 14(1): 7-19.
- Andrade RJ (2019) Landscape of Liver Injury from Herbal and Dietary Supplements in Europe, Latin America, and Asia. Clin Liver Dis (Hoboken) 14(2): 49-50.
- Yang LX, Liu CY, Zhang LL, Zheng ZW, Fan ZD, et al. (2017) Clinical characteristics of patients with drug-induced liver injury. Chin Med J (Engl) 130(20): 160-164.
- Takikawa H, Murata Y, Horiike N, Fukui H, Onji M, et al. (2009) Drug‐induced liver injury in Japan: An analysis of 1676 cases between 1997 and 2006. Hepatol Res 39(5): 427-431.
- Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, et al. (2012) A prospective nationwide study of drug‐induced liver injury in Korea. Am J Gastroenterol 107(9): 1380‐1387.
- Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S (2013) Incidence, presentation and outcomes in patients with drug‐induced liver injury in the general population of Iceland. Gastroenterology 144(7): 1419‐1425.
- Vega M, Verma M, Beswick D, Bey S, Hossack J, et al. (2017) The incidence of drug-and herbal and dietary supplement-induced liver injury: preliminary findings from gastroenterologist-based surveillance in the population of the state of Delaware. Drug Saf 40(9): 783-787.
- Navarro V, Avula B, Khan I, Verma M, Seeff L, et al. (2019) The Contents of Herbal and Dietary Supplements Implicated in Liver Injury in the United States Are Frequently Mislabeled. Hepatol Commun 3(6): 792-794.
- Blanck HM, Serdula MK, Gillespie C, Galuska DA, Sharpe PA, et al. (2007) Use of nonprescription dietary supplements for weight loss is common among Americans. J Am Diet Assoc 107(3): 441-447.
- Klontz KC, DeBeck HJ, LeBlanc P, Mogen KM, Wolpert BJ, et al. (2015) The role of adverse event reporting in the FDA response to a multistate outbreak of liver disease associated with a dietary supplement. Public Health Rep 130(5): 526-532.
- Smith TB, Staub BA, Natarajan GM, Lasorda DM, Poornima IG (2014) Acute myocardial infarction associated with dietary supplements containing 1,3-dimethylamylamine and Citrus aurantium. Tex Heart Inst J 41(1): 70-72.
- Roytman MM, Porzgen P, Lee CL, Huddleston L, Kuo TT, et al. (2014) Outbreak of severe hepatitis linked to weight loss supplement OxyELITE Pro. Am J Gastroenterol 109(8): 296-298.
- Foley S, Butlin E, Shields W, Lacey B (2014) Experience with OxyELITE Pro and acute liver injury in active duty service members. Military Med 59(12): 3117-3121.
- Heidermann LA, Navarro VJ, Ahmad J, Hayashi PH, Stolz A, et al. (2016) Severe acute hepatocellular injury attributed to OxyELITE Pro: a case series. Dig Dis Sci 61(9): 2741-2748.
- Johnston DI, Chang A, Viray M, Chatham-Stephens K, He H, et al. (2016) Hepatotoxicity associated with the dietary supplement OxyELIT ProTM - Hawaii, 2013. Drug Test Anal 8(3-4): 319-327.
- Chatham-Stephens K, Taylor F, Chang A, Peterson A, Daniel J, et al. (2017) Hepatotoxicity associated with weight loss or sports dietary supplements, including OxyELITE ProTM – United States, 2013. Drug Test Anal 9(1): 68-74.
- Miousse IR, Skinner CM, Lin H, Ewing LE, Kosanke SD, et al. (2017) Safety assessments of the dietary supplement OxyELITE Pro (new formula) in inbred and outbred mouse strains. Food Chem Toxicol 109(Pt 1): 194-209.
- Fontana RJ (2019) Severe Acute Hepatitis Attributed to the Herbal and Dietary Supplement OxyELITE Pro. Clin Liver Dis (Hoboken) 14(2): 45-48.
- Ferreira GSA, Watanabe ALC, Trevizoli NC, Jorge FMF, Diaz LGG, et al. (2020) Acute Liver Failure Caused by Use of Fat Burner: A CaseReport. Transplant Proc S0041-1345(19): 31709-31799.
- Dara L, Hewett J, Lim JK (2008) Hydroxycut hepatotoxicity: a case series and review of liver toxicity from herbal weight loss supplements. World J Gastroenterol 14(45): 6999-7004.
- Fong TL, Klontz KC, Canas-Coto A, Casper SJ, Durazo FA, et al. (2010) Hepatotoxicity due to hydroxycut: a case series. Am J Gastroenterol 105(7): 1561-1566.
- Khetpal N, Mandzhieva B, Shahid S, Khetpal A, Jain AG (2020) Not All Herbals are Benign: A Case of Hydroxycut-induced Acute Liver Injury. Cureus 12(2): e6870.
- Kothadia JP, Kaminski M, Samant H, Olivera-Martinez M (2018) Hepatotoxicity Associated with Use of the Weight Loss Supplement Garcinia cambogia: A Case Report and Review of the Literature. Case Reports Hepatol 2018: 6483605.
- Crescioli G, Lombardi N, Bettiol A, Marconi E, Risaliti F, et al. (2018) Acute liver injury following Garcinia cambogia weight-loss supplementation: case series and literature review. Intern Emerg Med 13(6): 857-872.
- Melendez-Rosado J, Snipelisky D, Matcha G, Stancampiano F (2015) Acute hepatitis induced by pure Garcinia cambogia. J Clin Gastroenterol 49(5): 449-450.
- Corey R, Werner KT, Singer A, Moss A, Smith M, et al. (2016) Acute liver failure associated with Garcinia cambogia use. Ann Hepatol 15(1): 123-126.
- Lunsford KE, Bodzin AS, Reino DC, Wang HL, Busuttil RW (2016) Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J Gastroenterol 22(45): 10071-10076.
- Sharma A, Akagi E, Njie A, Goyal S, Arsene C (2018) Acute Hepatitis due to Garcinia Cambogia Extract, an Herbal Weight Loss Supplement. Case Rep Gastrontest Med 2018: 9606171.
- Yousaf MN, Chaudhary FS, Hodanazari SM, Sittambalam CD (2019) Hepatotoxicity associated with Garcinia cambogia: A case report. World J Hepatol 11(11): 735-742.
- Rodriguez EA, Teixeira Yokoda R, Payton DE, Pai R, Byrne TJ (2019) Acute Hepatitis Secondary to the Use of Ilex paraguariensis (Mate Tea): A Case Report and Review of Literature. Case Reports Hepatol 2019: 8459205.
- Navarro VJ, Bonkovsky HL, Hwang SI, Vega M, Barnhart H, et al. (2013) Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci 58: 2682-2890.
- Yu Z, Samavat H, Dostal AM, Wang R, Torkelson CJ, et al. (2017) Effect of Green Tea Supplements on Liver Enzyme Elevation: Results from a Randomized Intervention Study in the United States. Cancer Prev Res (Phila) 10(10): 571-579.
- D'Agostinoa D, Cavalieri ML, Arcucci MS (2019) Severe hepatitis caused by green tea intoxication in a child. Case report. Arch Argent Pediatr 117(6): e655-e658.
- Oketch-Rabah HA, Roe AL, Rider CV, Bonkovsky HL, Giancaspro GI, et al. (2020) United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol Rep 7: 386-402.
- Stolz A, Navarro V, Hayashi PH, Fontana RJ, Barnhart HX, et al. (2019) Severe and protracted cholestasis in 44 young men taking bodybuilding supplements: assessment of genetic, clinical and chemical risk factors. Aliment Pharmacol Ther 49(9): 1195-1204.
- O Grundmann (2017) Patterns of Kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend 176: 63-70.
- Anwar M, Law R, Schier J (2016) Notes from the field: kratom (Mitragyna speciosa) exposures reported to poison centers – USA, 2010-2015. MMWR Morb Mortal Wkly Rep 65: 748-749.
- Schimmel J, Amioka E, Rockhill K, Haynes CM, Black JC, et al. (2020) Prevalence and description of kratom (Mitragyna speciosa) use in the United States: A cross-sectional study. Addiction.
- Tayabali K, Bolzon C, Foster P, Patel J, Kalim MO, et al. (2018) Kratom: a dangerous player in the opioid crisis. J Commun Hosp Intern Med Perspect 8(3): 107-110.
- Osborne CS, Overstreet AN, Rockey DC, Schreiner AD (2019) Drug-Induced Liver Injury Caused by Kratom Use as an Alternative Pain Treatment Amid an Ongoing Opioid Epidemic. J Investig Med High Impact Case Rep 7: 2324709619826167.
- Fernandes CT, Iqbal U, Tighe SP, Ahmed A (2019) Kratom-Induced Cholestatic Liver Injury and Its Conservative Management. J Investig Med High Impact Case Rep 7: 2324709619836138.
- Schimmel J, Dart RC (2020) Kratom (Mitragyna Speciosa) Liver Injury: A Comprehensive Review. Drugs 80(3): 263-283.
- Riverso M, Chang M, Soldwvila-Pico C, Lai J, Liu X, et al. (2018) Histologic characterization of Kratom Use-Associated Liver Injury. Gastroenterol Res 11(1): 79-82.
- Aldyab M, Ell PF, Bui R, Chapman TD, Lee H, et al. (2019) Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of the literature. Gastroenterol Res 12(4): 211-215.
- Prozialeck WC, Avery BA, Boyer EW, Grundmann O, Henningfield JE, et al. (2019) Kratom policy: The challenge of balancing therapeutic potential with public safety. Int J Drug Policy 70: 70-77.
- DEA (2016) Withdrawal of notice of intent to temporarily place mitragynine and 7-hydroxymitragynine into Schedule I. Fed Regist 81(198): 70652-70654.
- Prozialeck WC, Avery BA, Boyer EW, Grundmann O, Henningfield JE, et al. (2019) The challenge of balancing therapeutic potential with public safety. Int J Drug Policy 70: 70-77.
- Sethi R, Hoang N, Ravishankar DA, McCracken M, Manzardo AM, et al. (2020) Kratom (Mitragyna speciose): friend or foe? Prim Care Companion CNS Disord 22(1).
- https://appropriations.house.gov/sites/democrats.appropriations.house.gov/files/FY2020%20LHHS_Report.pdf.
- Vercammen KA, Koma JW, Bleich SN (2019) Trends in Energy Drink Consumption Among U.S. Adolescents and Adults, 2003-2016. Am J Prev Med 56(6): 827-833.
- Vivekanandarajah A, Ni S, Waked A (2011) Acute hepatitis in a woman following excessive ingestion of an energy drink: a case report. J Med Case Rep 5: 227.
- Apestegui CA, Julliard O, Ciccarelli O, Duc DK, Lerut J (2011) Energy drinks: another red flag for the liver allograft. Liver Transpl 17(9): 1117-1118.
- Huang B, Kunkel D, Kabany ME (2014) Acute Liver Failure Following One Year of Daily Consumption of a Sugar-Free Energy Drink. ACG Case Rep J 1(4): 214-216.
- Harb JN, Taylor ZA, Khullar V, Sattari M (2016) Rare cause of acute hepatitis: a common energy drink. BMJ Case Rep.
- Cattaneo D, Riva A, Filice C, Gervasoni C (2020) Liver Injury After Occasional Energy Drink Use in a Patient Living with HIV and Diabetes. Ann Pharmacother 54(3): 292-293.
- Uwaifo GI (2019) Beware Energy Drinks: A Case of a Toxic Triad Syndrome in a Diabetic Patient with Nonalcoholic Fatty Liver Disease. Am J Med Sci 358(4): 304-311.
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-distinguishing-liquid-dietary-supplements-beverages.
- Thiele E, Marsh E, Mazurkiewicz-Belzinska M, Halford JJ, Gunning B, et al. (2019) Cannabidiol in patients with Lennox-Gastaut syndrome: interim analysis of an open-label extension study. Epilepsia 60(3): 419-428.
- Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, et al. (2019) Long-term cannabidiol treatment in patients with Dravet syndrome: an open-label extension trial. Epilepsia 60(2): 294-302.
- S. Food and Drug Administration (2018) FDA briefing document: peripheral and central nervous system drugs advisory committee meeting (NDA 210365, Cannabidiol).
- S. Food and Drug Administration (2019) “FDA warns 15 companies for illegally selling various products containing cannabidiol as agency details safety concerns.” FDA, 25 Nov. 2019.
- Ewing LE, Skinner CM, Quick CM, Kennon-McGill S, McGill MR, et al. (2019) Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules 24(9): 1694.
- Ewing LE, McGill MR, Yee EU, Quick CM, Skinner CM, et al. (2019) Paradoxical Patterns of Sinusoidal Obstruction Syndrome-Like Liver Injury in Aged Female CD-1 Mice Triggered by Cannabidiol-Rich Cannabis Extract and Acetaminophen Co-Administration. Molecules 24(12): 2256.
- Parvez MK, Rishi V (2019) Herb-drug interactions and hepatotoxicity. Curr Drug Metab 20(4): 275-282.
- https://www.fda.gov/news-events/press-announcements/fda-warns-15-companies-illegally-selling-various-products-containing-cannabidiol-agency-details.
- Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT (2013) Why US adults use dietary supplements. JAMA Intern Med 173(5): 355‐361.
- Foley H, Steel A, Cramer H, Wardle J, Adams J (2019) Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep 9(1): 1573.
- Rao T, Liu YT, Zeng XC, Li CP, Ou-Yang DS, et al. (2020) The hepatotoxicity of Polygonum multiflorum: the emerging role of the immune-mediated liver injury. Acta Pharmacol Sin.
- Avigan MI, Mozersky RP, Seeff LB (2016) Scientific and regulatiory perspecxtives in herbal and dietary supplement associated hepatotoxicity in the United States. Int J Mol Sci Mar 17(3): 331.
- Brown AC (2017) An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem Toxiciol 107(Pt A): 449-471.
- Higgins JP, Tuttle TD, Higgins CL (2010) Energy beverages: content and safety. Mayo Clin Proc 85(11): 1033.
- Schffellner S, Stadlbauer V, Sereinigg M, Mìller H, Högenauer C, et al. (2017) Niacin-associated acute hepatotoxicity leading to emergency liver transplantation. Am J Gastroenetrol 112(8): 1345-1346.
- Crippa JA, Guimarães FS, Campos AC, Zuardi AW (2018) Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol 9: 1-16.






























