XIAP Deficiency in Refractory Crohn´s Disease: A Case Report in Brazil
Vivian Regina Guzela1*, Carlos Walter Sobrado Júnior1, Sérgio Carlos Nahas1, Ivan Cecconello1 and Dewton de Moraes Vasconcelos2
1Departamento de Gastroenterologia, Hospital das Clínicas da Faculdade de Medicina – Universidade de São Paulo, Brazil
1Faculdade de Medicina – Universidade de São Paulo / Instituto de Medicina Tropical de São Paulo, Brazil
Submission: May 13, 2019; Published: May 22, 2019
*Corresponding author:Vivian Regina Guzela, Hospital das Clínicas da Faculdade de Medicina – Universidade de São Paulo/ Departamento de Gastroenterologia, Av. Dr. Enéas de Carvalho Aguiar, 255. Cerqueira César – São Paulo/SP 05403-000, Brazil
How to cite this article: Vivian R G, Carlos W S J, Sérgio C N, Ivan C, Dewton d M V. XIAP Deficiency in Refractory Crohn´s Disease: A Case Report in 002 Brazil. Adv Res Gastroentero Hepatol. 2019; 13(1): 555853. DOI: 10.19080/ARGH.2019.12.555853.
Abstract
Introduction: A case report to highlight the association between refractory Crohn´s disease and X-linked inhibitor apoptosis protein deficiency syndrome, a rare genetic disease that occurs in males. Lymphoproliferative syndrome and splenomegaly associated or not to Crohn´s disease are common manifestations. Case presentation: A 28 years-old-man presenting perianal fistula, associated to intense abdominal pain and chronic diarrhea with mucus and blood, which didn´t respond to the combined treatment (surgery and biologic agents). In his youth, he had presented severe mononucleosis. Besides the common findings in inflammatory disease, there was hypogammaglobulinemia and tendency to low levels of natural killer-T cells-, with genetic evaluation confirming X-linked inhibitor apoptosis protein deficiency syndrome. The patient was referred to allogeneic stem cell transplantation. However, after eighteen days of bone marrow infusion and during the engraftment stage, the patient had developed pneumonia and sepsis resulting in death. Conclusion: Severe refractory Crohn’s disease presents an important complementary diagnosis: XIAP deficiency. It should be mainly considered in male patients with a history of severe infections and lymphoproliferative syndrome.
Keywords: Crohn´s disease; XIAP deficiency; Lymphoproliferative syndrome; Bone marrow infusion; Protein deficiency syndrome; Hypogammaglobulinemia; Inflammatory disease; Allogeneic stem cell transplantation; Natural killer-T cells
Abbrevations: DC: Crohn´s Disease; IBD: Inflammatory Bowel Disease; NOD: Nucleotide-Binding Oligomerization Domain-Containing Protein; CRP: C-Reactive Protein; MRI: Magnetic Resonance Imaging; CT: Computed Tomography; Anti-TNF: Anti-Tumor Necrosis Factor; XIAP: X-Linked Inhibitor of Apoptosis; HLH: Hemophagocytic Lymphohistiocytosis; XLP: X-Linked Lymphoproliferative Disease
Introduction
Crohn´s disease
Crohn’s disease (CD) is an inflammatory bowel disease (IBD), associated with a disproportionate inflammatory response, which may present periods of remission and exacerbation of unknown etiology. It may affect both genders proportionally, with a bimodal age distribution in peaks between 20 and 40 years and another between 50 and 60 years. Its incidence is increasing mainly in urban and developed countries [1]. Some environmental factors including smoking, recurrent use of nonsteroidal anti-inflammatory drugs and a history of appendectomy may predispose to CD as well as microbiota alterations. Another important factor that contributes to the development of the disease is the loss of the intestinal mucosal immune system homeostasis attributed to thedefects of the epithelial barrier and the apoptosis of the T lymphocytes. Alterations in genes related to autophagy (ATG16L1 and IRGM) and to NOD-2 receptor (nucleotide-binding oligomerization domain-containing protein 2, an intracellular pattern recognition receptor), are involved in this deregulation, as well as other genes (IL23R, HLA-II, STAT3, JAK2, LRRK2) [2,3].
Clinical manifestations may vary depending on the extension, severity, and location of the disease. The most common symptoms are abdominal pain and chronic diarrhea, with or without pathological products (blood and mucus). Nonspecific symptoms such as malaise, weight loss, fever and hyporexia may be part of the clinical condition, as well as extra-intestinal manifestations (articular, cutaneous, ocular and dermatological). Approximately one-third of the patients may present with perianal manifestations, like deep fissures and inflammatory skin tags, anal stricture and development of complex fistulas, abscesses or ulcers [4]. Laboratory tests may reveal nonspecific signs as iron deficiency anemia, vitamin B12 deficiency, increased inflammatory markers such as C-reactive protein (CRP), erythrocyte sedimentation rate and thrombocytosis. Detection of antibodies such as anti - Saccharomyces cerevisiae or antibodies against the porin C of the outer membrane of Escherichia coli are low specificity tests [5]. The increased measurement of lactoferrin and faecal calprotectin may be associated with the diagnosis of Crohn’s Disease and can evaluate disease activity with an excellent endoscopic correlation [6].
Deep punctate or serpiginous ulcers, with a “cobble stone” appearance, usually with besides areas of normal mucosa are observed in the ileocolonoscopy. The collection of two fragments in five different regions, including the ileum and rectum, may improve the diagnosis in less obvious cases. Upper digestive endoscopy and endoscopic capsule evaluations are reserved for cases with unspecific symptomatology or diagnostic difficulty by the most commonly used methods. The histopathological analysis may reveal a transmural inflammatory process with lymphoplasmacytic infiltrate, associated with crypt irregularities and non-caseous granulomas [7].
Magnetic resonance imaging (MRI) and computed tomography (CT) of the abdomen may show parietal thickening, mesenteric fat blurring, adenopathy, collections, stenosis, and fistulas. Endoanal ultrasonography may also assist in the more detailed study of perianal fistulae, however stenosis commonly present in these
patients is a limitation of the method.
The treatment of these patients includes two strategies: stepup and top-down. The first, most commonly used, is based in inducing remission of the disease using progressive scale medications without the inclusion of corticosteroids in maintenance. The second, based on the search for endoscopic remission in order to prevent complications of inflammatory disease, provides for the use of more systemic spectrum medications such as biological therapy and immunosuppressant drugs as first choice [8].Among the different classes of medications used to treat CD, antibiotics are indicated for septic complications, bacterial overgrowth and perianal disease. 5-aminosalicylic acid derivatives (sulfasalazine and mesalazine) have benefits in colon disease, but little effect on other topographies. Systemic glycocorticoids are reserved for the exacerbations, limited to 12 weeks at the usual dose of 1mg/kg/day of prednisone, due to the adverse effects secondary to prolonged use (osteopenia, Cushing’s syndrome and hyperglycemia).
Immunosuppressants are mainly used in maintenance therapy because they present a slow onset of action, but with the advantage of allowing withdrawal of corticosteroids in dependent patients and should be maintained for long periods to reduce the risk of relapse. Biological therapy with the use of anti-tumor nenecrosis factor (anti-TNF) agents such as infliximab, adalimumab and certolizumab has shown an effective response in the induction and maintenance of these patients, increased by the association with immunosuppressants. Other medications of this class such as vedolizumab and ustekinumab (anti-47 integrin and anti-interleukin 12/23, respectively) are options in the failure of the medications listed previously. The greatest risk of the use of these drugs is predisposition to opportunistic infections.
Surgical treatment should be an option in specific cases, such as absence of response to the treatments, corticosteroid dependence, obstructive stenosis, intestinal perforation, fistulas, hemorrhages, abdominal sepsis and complex perianal disease. The surgeries should always be as conservative as possible, because of high recurrence rate and diffuse involvement of the gastrointestinal tract [4].
XIAP deficiency syndrome
X-Linked Inhibitor of Apoptosis (XIAP) deficiency is a primary rare immunodeficiency, described for the first time in 2006 [9]. Until now, there are approximately seventy reported cases.
Despite being an anti-apoptotic molecule, XIAP is also involved in other mechanisms like inflammation and the innate immune response. It´s necessary to regulate signaling of the receptors NOD-1 e NOD-2, which detect bacterial infections. Deficient XIAP cells like natural killer-T (NKT) lymphocytes in the mucosa are more prone to apoptosis, resulting in imbalance of the immune response of these individuals [10]. Moreover, the deregulated NOD-1 and NOD-2 signaling lead to an exaggerated inflammatory response in the intestine and severe gastrointestinal manifestations.
Patients with XIAP Deficiency were previously known as X-linked lymphoproliferative disease carriers (XLP), but with a normal sequence of SH2D1A (SAP), (XLP1). Both defects predispose to hemophagocytic lymphohistiocytosis (HLH), however, XIAP deficiency, also known as XLP-2 apparently don´t have the same risk to develop lymphomas [11].
XIAP deficiency may evolve with HLH, triggered by Epstein- Barr infection, Herpes Virus – 6 (HSV-6) or cytomegalovirus and splenomegaly (54 and 57% of the cases, respectively). Clinical manifestations like inflammatory bowel disease may be the first sign up to 30% of the patients, being the biologic behavior and the histology identical to CD [10,12]. Other phenotypes like arthritis, abscesses, erythema nodosum, uveitis and nephritis occur in 7% of the XIAP deficient patients.
The XIAP gene has six exons and more than fifty different mutations have been shown until now, which may cause complete lack of protein expression or just its reduced activity, justifying different degrees of severity of the disease [13]. Heterozygous women for XIAP mutations are usually healthy and asymptomatic, but three cases of female patients with deficiency of XIAP expression have been described, two of them had inflammatory bowel disease and other with HLH associated to Epstein-Barr infection
Flow cytometry to the analysis of the reduced expression of XIAP allows the screening in suspect patients, however it is not capable of identify all the affected. The genetic sequencing with mutation identification remains the gold standard diagnostic test, despite it cannot presume prognostic [10].
Lymphoproliferative disease, inflammatory bowel disease and splenomegaly, with or without hypogammaglobulinemia, in men with history of severe mononucleosis, is highly suspect for XIAP deficiency [14].
Prescription of the therapeutic classes normally used in IBD is not a consensus to patients with XIAP deficiency. Allogeneic stem cell transplant, which reestablishes the XIAP expression is a therapeutic option in some cases with severe intestinal manifestations and may prevent the evolution to HLH [15], despite some studies hadn´t shown advantage in morbidity or mortality [16]. The intravenous infusion of immunoglobulin may be part of the treatment. This disease has reserved prognostic and high mortality associated.
So, in this case report, we emphasize the importance of the immunological and genetic evaluation of those patients with CD since the childhood, with poor evolution, refractory to the gold standard treatment (biologics and immunosuppressant) and with family history of IBD because this group may reveal genetic monogenic diseases. Especially in male patients, XIAP deficiency must be considered.
Case Report
A caucasian male, 28 years-old patients, developed perineal and pelvic pain for one year, evolving to fistulas in perianal region, with continuous pyogenic secretion. Diffuse opioid-refractory abdominal pain emerged after the perineal manifestations, associated with tenesmus and diarrhea, up to 30 times daily, with bloody stool.
In his youth, at age 15, he had presented severe mononucleosis with myocarditis, hepatitis and meningoencephalitis. There was no evidence of familiar history of IBD or other relevant diseases.
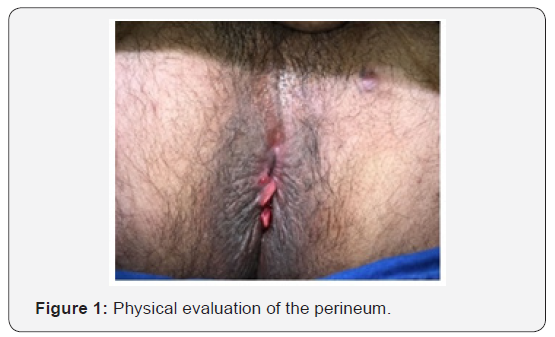
During the physical evaluation, the abdomen was flat, diffusely painful to superficial and deep palpation, without masses andabrupt decompression absent. In the perineum, there was a fistula approximately 3 cm distant from the anal verge, in the left anterolateral region and posterior inflammatory skin tags with a poor delimited ulcer (Figure 1).
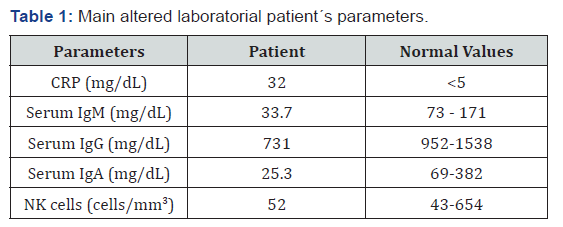
A complete blood count revealed normal level of leukocytes and platelets, normal renal function and high CRP concentrations. Due to the severity of the condition and the prior history, the levels of immunoglobulins were measured, considering associated immunodeficiency (Table 1).
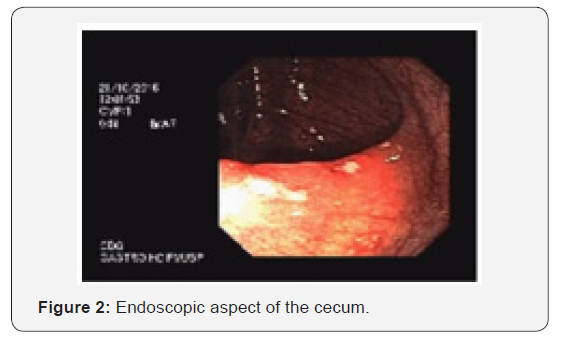
The colonoscopy revealed diffuse hyperemia, since the ileum until the rectum, with multiple aftoid ulcers (Figure 2), most prominent in the distal tract and the pathological evaluation showed active chronic colitis without granulomas. Upper digestive endoscopy presented with mucosal erosions covered by fibrin in the gastric antrum (chronic gastritis without specific findings).
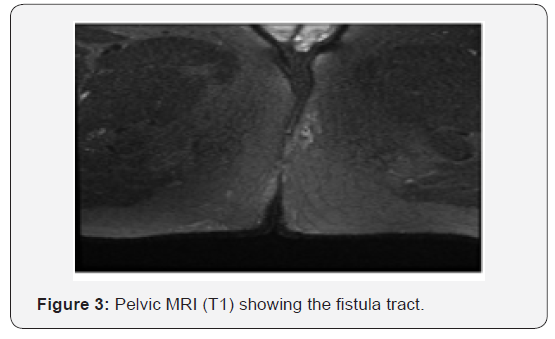
The imaging analysis revealed in the MRI: trans-sphincteric fistulous tract with left anterolateral mucosal orifice, transversing the ischioanal and subcutaneous fossa in the left gluteus, until externalized in this region at 2, 5cm of the anal verge, associated with small amount of liquid, without abscess (Figure 3).
The intestinal transit evidenced stomach, duodenum, jejunum, ileum with normal distribution (Figure 4).
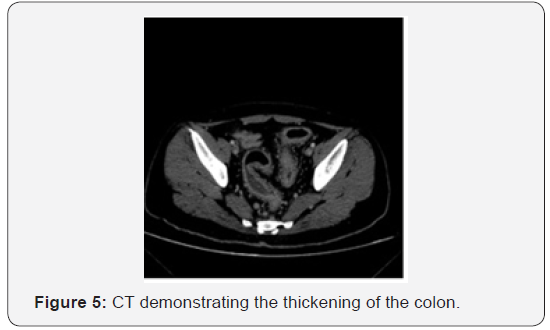
The CT presented diffuse parietal thickening of the descending and sigmoid colon, with engorgement of the pericolic vessels and densification of the regional adipose planes, associated with lymph node enlargement up to 1,2cm on the largest axis (Figure 5).
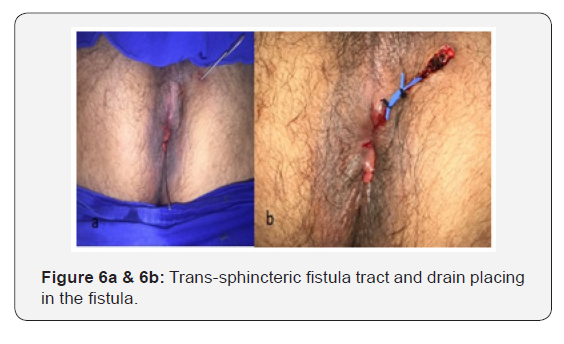
Intravenous infusion of immunoglobulin (1x/monthly) and subcutaneous adalimumab (14/14 days) were prescribed, after the recommended induction doses of the anti-TNF agent (160mg in week 0, 80mg in the week 2 and 40 mg in the week 4), and the surgical treatment of the fistula tract was done with drain placing (Figure 6a &b) and biopsy of the tract.
Despite the treatment, the abdominal pain, mainly related to the meals persists and become refractory to opioids, resulting in the need of parenteral diet due to the inability of the patient to accept oral diets and medications. The dose optimization of adalimumab to weekly injections did not show relief of the symptoms or reverse of the inflammatory manifestations. Even with parenteral support, prescription of anti-TNFα agent, surgery and analgesia, the patient´s clinical condition worsened.
With the genetic sequencing proving XIAP deficiency and in the face of the refractoriness and severity of the clinical condition, the patient was selected for allogeneic bone marrow transplantation. The procedure led to an initial improvement of the symptoms; however, after eighteen days of the transplantation during the engraftment period of stem cells, the patient presented a liver venocclusive disease with fast evolution (<24 hours) to sepsis and death, despite the prompt therapy of the infection
Discussion
This case report, of a young man with IBD (ileum-colo-anal manifestations) with a severe and refractory evolution despite the gold standard treatment and a history of atypical infection evolution in his youth, shows the importance to consider the immunological and genetic evaluation of this group. The careful attention to this group of patients allows early identification and correct approach of patients with genetic syndromes compromising the gastrointestinal tract.
When a patient is eligible to receive the “top-down” strategies for IBD, it is precisely at preventing it from evolving with sequelae and severe complications such as stenosis, perforations, fistulas and perineal ulcers, in addition to extra-intestinal manifestations. Thus, this case report suggests that a multi professional team should follow those high-risk group patients, allowing the precocious diagnosis and the assertive treatment
Even with a great development of the IBD therapeutic arsenal, some cases simply do not show any improvement of the clinical condition with the correct treatment and drugs or therapeutic classes interchange. Specifically related to the biologic agents, the current main discussion focus in resistance associated to immune complexes development, turning necessary the measure the serum levels of anti-drugs antibodies. However, for patients that not respond since the first prescriptions, we should consider genetic syndromes like XIAP deficiency as a possible diagnosis, instead of insisting in medications interchange with the hypothesis of lack of response due to drug resistance
In conclusion, this case report highlights the importance to think about additional genetic diagnosis in IBD patients and evidences that even in a country with lack of resources is possible to make differential diagnosis, even though the process is slower than the ideal due to the difficulty of accessing more specific analyzes such as genetic sequencing. Until now, the small number of described cases causes the impression that it may be an exception diagnosis, but the identification and the assertive approach of this special group can be done in referential centers, with a multi professional teamwork.
Ethics Approval and Consent to Participate
This case report was submitted to the ethical committee Comitê de Ética para Análise de Projetos de Pesquisa (CAPPESQ) of the Hospital das Clínicas – Faculdade de Medicina and to Plataforma Brasil, with number approval 03509818.0.0000.0068
Because of the patient´s outcome, the committee exempted the authors to obtain the informed consent of the patient and his family.
Availability of Data and Materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study, because it is a case report in which all relevant exams and patient´s information are available in the article itself
Author´s Contribution
Carlos Walter Sobrado Junior wrote this article e was responsible for the treatment of the patient, especially for the surgical approach.
Vivian Regina Guzela gathered and reviewed the clinical data of the case report and wrote this article
Dewton de Moraes Vasconcelos reviewed this article and was responsible for the treatment of the patient, mainly the immunological approach.
Sérgio Carlos Nahas reviewed this article and was responsible for provide part of the institutional resources as a chief of the Colon and Rectal Surgery Division.
Ivan Cecconello reviewed this article and was responsible for provide part of the institutional resources as a chief of the Gastroenterology Department.
References
- Ferré MPB, Boscá-Watts MM, Pérez MM (2018) Crohn´s Med Clin 151(1): 26-33.
- Coulombe F, Divangahi M, Veyrier F, de Léséleuc L, Gleason JL, et al. (2009) Increased NOD-2 mediated recognition of N-glucolyl myramyl dipeptide. J Exp Med 206(8): 1709-1716.
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, et al. (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491(7422): 119-124.
- Feuerstein JD, Cheifetz AS (2017) Crohn disease: epidemiology, diagnosis and management. Mayo Clin Proc 92(7): 1088-1103.
- Bossuyt X (2006) Serologic markers in inflammatory bowel disease. Clin Chem 52(2): 171-181.
- Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, et al. (2015) C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: A systematic review and meta-analysis. Am J Gastroenterol 110(6): 802-819.
- Magro F, Langner C, Driessen A, Ensari A, Geboes K, et al. (2013) European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 7(10): 827-851.
- Khanna R, Bressler B, Levesque BG, Zou G, Stitt LW, et al. (2015) Early combined immunosuppression for the management of Crohn’s disease (REACT): A cluster randomised controlled trial. Lancet 386(10006): 1825-1834.
- Rigaud S, Fondanéche C, Lambert N, Pasquier B, Mateo V, et al. (2006) XIAP deficiency in humans causes na X-linked lymphoproliferative syndrome. Nature 444(7115): 110-114.
- Aguilar C, Latour S (2015) X-linked Inhibitor of Apoptosis Protein Deficiency: More than an X-linked Lymphoproliferative Syndrome. J Clin Immunol 35(4): 331-338.
- Pachlopnik-Schmid J, Canioni D, Moshous D, Touzot F, Mahlaoui N, et al. (2011) Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/ XIAP deficiency). Blood 117(5): 1522-1529.
- Zeissig Y, Petersen BS, Milutinovic S, Bosse E, Mayr G, et al. (2014) XIAP variants in male Crohn’s Gut 64(1): 66-76.
- Damgaard RB, Fiil BK, Speckmann C, Yabal M, Stadt UZ, et al. (2013) Disease-causing mutations in the XIAP BIR2 domain impair NOD2-dependent immune signalling. EMBO Mol Med 5(8): 1278-1295.
- Marsh RA, Bleesing JJ, Filipovich AH (2010) Using flow cytometry to screen patients for X-linked lymphoproliferative disease due to SAP deficiency and XIAP deficiency. J Immunol Methods 362(1-2): 2-9.
- Nielsen OH, LaCasse EC (2016) How genetic testing can lead to targeted management of XIAP deficiency-related imflammatory bowel disease. Genet Med 19(2): 133-143.
- Hawkey CJ, Allez M, Clark MM, Labopin M, Lindsay JO, et al. (2015) Autologous Hematopoetic Stem Cell Transplantation for Refractory Crohn Disease - A Randomized Clinical Trial. JAMA 314(23): 2524-2534.






























