Usefulness of Virtual Chromoendoscopy with Optical Enhancement in Everyday Clinical Practice
Gian Eugenio Tontini1*, Alessandro Rimondi1, Helmut Neumann2, Sauid Ishaq3,4, Flaminia Cavallaro1, Maurizio Vecchi1,5 and Luca Pastorelli1,5
1Gastroenterology & Digestive Endoscopy Unit, IRCCS Policlinico San Donato, Italy
2First Medical Department, University Medical Center Mainz, Germany
3Department of Gastroenterology, Dudley Group Hospitals & Birmingham City University, United Kingdom
4St. George's University, Grenada, West Indies
5Department of Biomedical Sciences for Health, University of Milan, Italy
Submission: November 01, 2017; Published: November 16, 2017
*Corresponding author: Gian Eugenio Tontini, MD, PhD, Gastroenterology & Digestive Endoscopy Unit, IRCCS Policlinico San Donato, Via Morandi 30, 20097, San Donato Milanese [MI], Italy Tel: 39 0252774652; Fax: 390252774655; Email: gianeugeniotontini@gmail.com
How to cite this article: Gian E T, Alessandro R, Helmut N, Sauid I, Flaminia C, et al. Usefulness of Virtual Chromoendoscopy with Optical Enhancement in Everyday Clinical Practice. Adv Res Gastroentero Hepatol 2017; 7(4): 555719. DOI: 10.19080/ARGH.2017.07.555719
Abstract
Optical Enhancement [OE] is a novel endoscopic technology, which combines digital and optical dye-less chromoendoscopy to improve the visualization of both the mucosal surface and vascular pattern in real time. Whether this advanced endoscopic imaging technique may improve the detection and the endoscopic definition of inflammatory, dysplastic and pre-neoplastic lesions is still unknown. Hereby, we evaluated for the first time the impact of OE technology in every-day clinical practice based on a consecutive series of unselected patients referring for esophago- gastro-duodeno-scopy or colonoscopy at a tertiary referring center either for inflammatory or oncologic gastro-intestinal disorders.
Each patient received an endoscopic inspection using high-definition and i-scan digital chromoendoscopy. Subsequently, each segment has been further evaluated employing the OE technology using either the OE-mode 1, or the OE-mode 2. An experienced gastrointestinal pathologist reviewed all biopsy specimens placed in separate vials [i.e., OE-targeted, i-scan-targeted, random biopsies] in a blind fashion.
Twenty endoscopic procedures were consecutively performed for several indications, as part of a routine diagnostic work-up. In four cases [20%], the use of OE technology improved the endoscopic visualization of subtle lesions, thereby leading to a different clinical management in three patients [15%] affected by mucosa-associated T lymphoma, systemic vasculitis and BARGHett's oesophagus.
This initial experience suggests that the OE technology improves the endoscopic visualization and characterization of inflammatory, neoplastic and pre-neoplastic digestive disorders in everyday clinical practice. Large studies addressing a formal comparison between this novel endoscopic technology and previous dye-less chromoendoscopic techniques are required.
Keywords: Optical enhancement; Dye-less chromoendoscopy; Virtual chromoendoscopy; Optical chromoendoscopy; Digital chromoendoscopy; NARGHow-band imaging; I-scan; BARGHett's Oesophagus; Mucosa-associated T lymphoma; Ulcerative colitis
Core Tip
Optical Enhancement [OE] is a novel endoscopic technology, which combines digital and optical dye-less chromoendoscopy to improve the visualization of both the mucosal surface and vascular pattern in real time. We report the first experience with OE in a consecutive series of unselected patients receiving oesophago- gastro-duodeno-scopy or colonoscopy either for inflammatory or oncologic disorders. An improved endoscopic management was clearly observed in 15% procedures, and notably for mucosa- associated T lymphoma, BARGHett's oesophagus, and vasculitis. OE stands out as a promising everyday practice tool for either endoscopic detection or characterization of inflammatory and neoplastic disorders.
Introduction
With the advent of high-definition endoscopic imaging, the visualization of mucosal surface significantly improved the advent of high-definition endoscopic imaging, the visualization of mucosal surface significantly improved, providing a sharper and richer detail, thus allowing an easier detection of gastrointestinal lesions. Nonetheless, standard white-light endoscopy may miss several flat lesions, only advisable for subtle changes affecting either the surface or the vascular pattern [1]. Dye-based chromoendoscopy with either methylene blue or indigo carmine can remarkably improve the detection of surface abnormalities but it has a series of practical limitations and almost no impact on vessels' characterization [2]. Various dye-less chromoendoscopic techniques such as NARGHow Band Imaging (NBI, Olympus, Tokyo, Japan), Fujinon Intelligent Color Enhancement (FICE, Fujifilm, Tokyo, Japan), Storz Professional Image Enhancement Systems [SPIES, Karl Storz GmbH & Co. KG, Tuttlingen, Germany), and i-scan (Pentax, Tokyo, Japan) have further enriched the mucosal picture of details, contrast and colors, leading to many concrete diagnostic improvements [3-9]; indeed, these techniques are easier to handle, compared to dye- based chromoendoscopy, because already integrated in modern endoscopic systems. While, FICE and i-scan are based on a digital post-processing of acquired images that results in an enhanced tissue contrast, NBI uses optical lenses to nARGHow the bandwidth of spectral transmittance, thereby unveiling the mucosal and submucosal blood vessels [10]. Recently, the striking technological progress in the field of digestive endoscopy has brought to a second generation of dye-less chromoendoscopic techniques, enabling an improved visualization of both the mucosal surface and vascular pattern in real time. The Blue Laser Imaging system [LASEREO video processor; Fujifilm Co., Tokyo, Japan), first introduced in 2013 [11], trades on two monochromatic lasers: a 410nm laser to visualizes vascular micro architecture (like NBI), and a 450nm laser to provide the bright and clear view obtained with conventional xenon light sources. More recently, the Optical Enhancement technology (Optivista EPK-i7010 video processor; Pentax, Tokyo, Japan) was developed to allow a combination of digital (i.e., by signal processing, like i-scan) and optical (i.e., by optical filters that limit the spectral characteristics of the illumination light, like NBI) chromoendoscopy [12-14].
Hence, we describe for the first time the impact of Optical Enhancement technology [OE] in a consecutive unselected series of patients undergoing endoscopic examinations for suspected gastrointestinal disorders.
Methods
The OE technology has been tested at IRCCS Policlinico San Donato by one expert endoscopist without previous experience with the use of OE technology between July 22nd and August 11th 2016. All patients provided a written informed consensus and received a conscious sedation with i.v. midazolam ±pethidine. This study was cARGHied out in accordance with the World Medical Association Declaration of Helsinki adopted in 1964 incorporating all later amendments.
Initially, a high-definition (HD) endoscope was advanced using standard white-light imaging. On withdrawal, all parts were carefully inspected using i-scan digital chromoendoscopy. Subsequently, each segment has been further evaluated employing the OE technology using either the OE mode 1, or the OE mode 2. Briefly, the OE mode 1 uses light emission at 415nm and 540nm, corresponding to the main wavelengths of spectral transmission for hemoglobin absorption, to maximize the visualization of mucosal vessels. The optical filter of OE mode 2 also adds a red wavelength light source, thereby improving the overall image brightness [12,13].
Multiple biopsies were performed as per routine clinical practice, according to patients' history and endoscopic findings. Distinct biopsies were taken from
a) Macroscopically normal mucosa,
b) Abnormal mucosa according to i-scan endoscopic imaging, as well as from
c) Any subtle lesions revealed by OE only. All specimens were placed in separate vials and reviewed by an experienced gastrointestinal pathologist in a blind fashion.
Results
Twenty endoscopic procedures, including oesophago-gastro- duodeno-scopies [EGDSs] and colonoscopies, were consecutively performed for several indications, as part of the routine diagnostic work-up, in an outpatient or inpatient setting. Among these, the use of OE technology has been associated with an improved endoscopic visualization as compared to HD with i-scan imaging in the following four cases. Notably, the additional findings revealed only using the OE technology had a concrete impact on further clinical management in three of them (i.e., case 1, 2, and 4).
Case 1
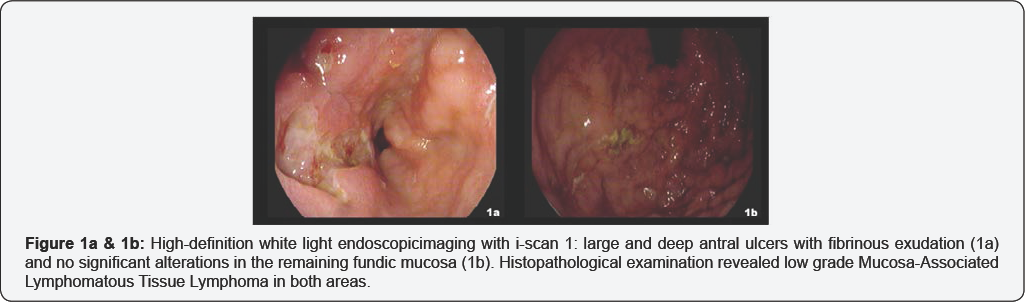
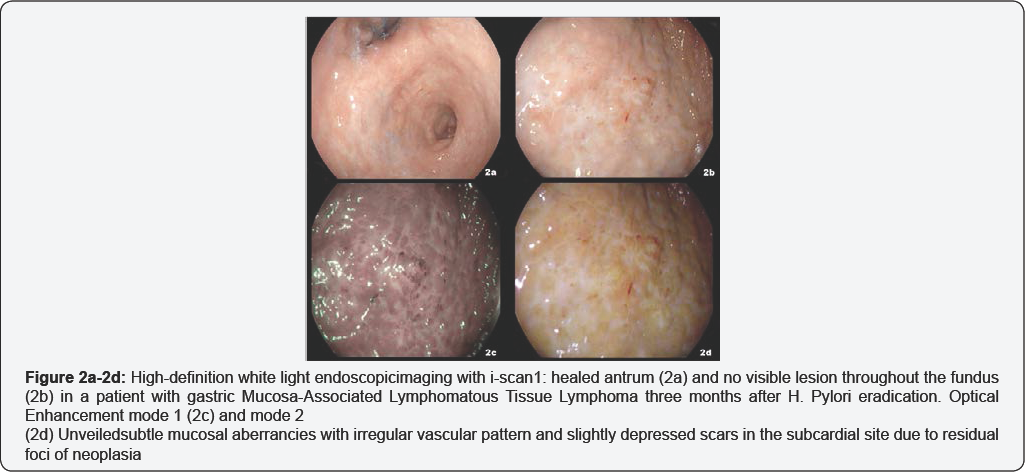
We first evaluated OE technology in a 50-year-old male with a history of gastric Mucosa-Associated Lymphomatous Tissue Lymphoma (MALToma). Four months before, the patient underwent an EGDS for dark faeces showing multiple large and deep ulcers located in antrum (Figure 1a). No additional significant lesion was visible using HD endoscopy with i-scan imaging in the residual gastric mucosa (Figure 1b). Four random biopsies were performed from any topographic gastric sites (antrum, lesser and greater curvature; incisura angularis; body, lesser and greater curvature; fundus) [15] and the histopatological evaluation, including immune is to chemistry demonstrated a low-grade gastric MALToma located in both the antrum and fundus. Three months after Helicobacter Pylori eradication, the endoscopic control based on HD plus i-scan imaging showed a healed antrum and no visible lesion throughout the fundus (Figure 2a & 2b). OE was employed revealing subtle mucosal aberrancies with irregular vascular pattern and slightly depressed scars in the subcardial site (Figure 2c & 2d, Video 1). Consistently, two OE-targeted samples were collected from this area in addition to four random biopsies from any topographic gastric sites. The histological and immune histochemical assessment confirmed the persistence of low-grade gastric MALToma on the OE-targeted biopsies but only mild chronic inflammation in the remaining specimens.
Case 2
A 19-year-old male patient, with a recent history of pyodermatitis, purpuric rashes at inferior limbs, skin ulcers, weight loss, and recurrent joint and abdominal pain was referred to our IBD center. Despite such clinical presentation, the presumed diagnosis of a systemic rheumatologic disease was not supported by an extensive clinical work-up, thereby leading to a gastroenterological consultancy for the clinical suspicious of Crohn's disease with extra- intestinal manifestations.


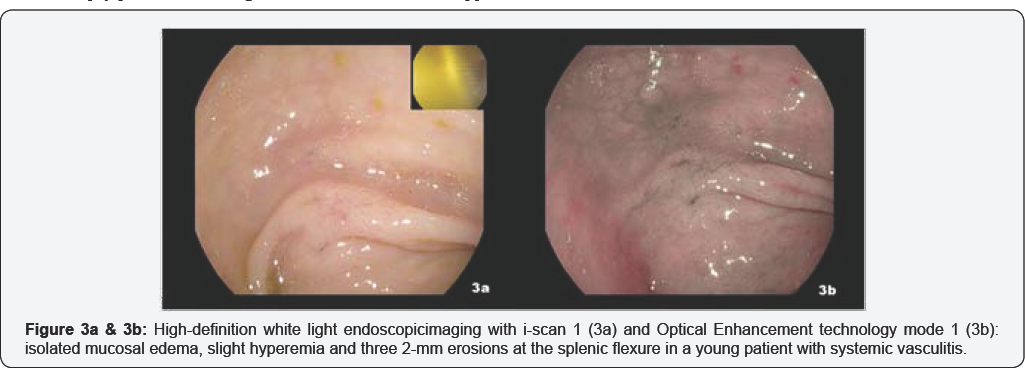
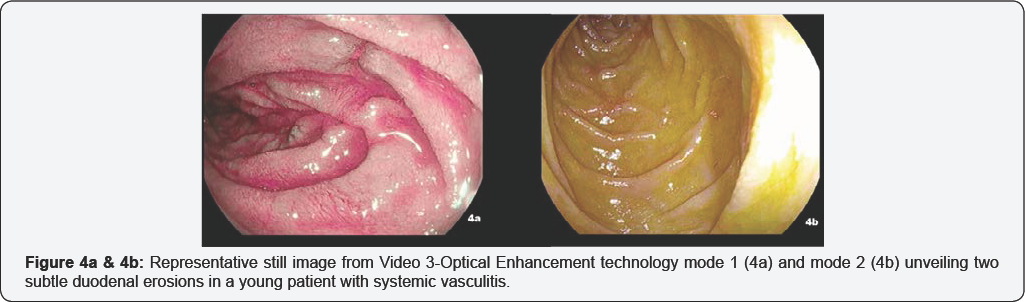
The ileo-colonoscopy reveals no mucosal abnormality under HD white-light imaging and i-scan. During the endoscope withdrawal, OE mode 1 technology identified isolated mucosal edema, slight hyperemia and three 2mm erosions at the splenic flexure (Figure 3a & 3b, Video 2). The following EGDS showed a normal appearing mucosa under HD white-light imaging and i-scan mode 1. While OE was engaged, two small 3mm erosions surrounded by a scARGHed mucosa appeared in the descending duodenum [Figure 4a & 4b, Video 3]. Histopathology reveals no microscopic aberrancy on random biopsy performed throughout both ileo-colonic and upper GI inspection. Remarkably, OE-targeted biopsy from colon and duodenum documented several microscopic changes consistent for a possible GI involvement of a systemic vasculitis.
These results benefited the ERCP team to proceed with the procedure if indicated, but to be more cautious during the procedure in those 82 patients with the less serious and non obstructive pathologies, while led to delay the procedure in the 32 patients with peptic ulcerations and benign obstructive lesions waiting improvement with appropriate therapy& to consider canceling the procedure in the 3 patients with malignant gastric outlet obstruction & search for other management options.

Case 3
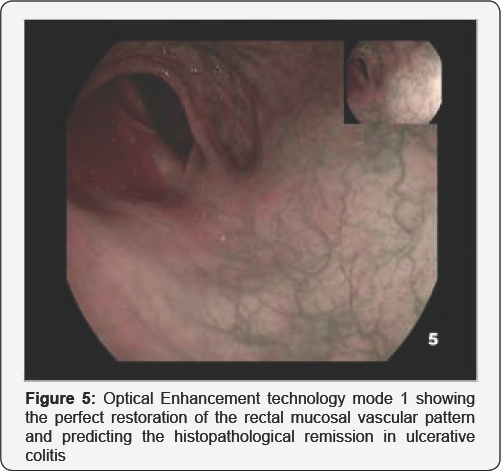
A 47-year-old male patient affected by a left-side ulcerative colitis was referred to colonoscopy for assessment of inflammatory disease activity and mucosal healing following an induction therapy with oral and topic mesalamine. No mucosal lesions were detected using HD white-light imaging and i-scan. The OE mode 1 technology clearly revealed a normal mucosal vascular pattern throughout the entire colon and rectum [Figure 5]. Consistently, the subsequent histopathological analysis based on multiple random biopsy confirmed a quiescent colitis with intact surface epithelium, normal crypt architecture and no inflammatory infiltrate in lamina propria.
Case 4
Finally, we used OE for the evaluation of a 62-year-old male patient with a history of gastro-esophageal reflux disease and atrophic gastritis. The EGDS with HD white-light imaging and i-scan showed multiple slight lesions above the gastroesophageal junction, including erosions and columnar appearing mucosa. By employing OE mode 1, the columnar appearing mucosa acquired a sharper superficial pattern and more distinct margins. The OE mode 2 provides similar effect in distinguishing the columnar appearing mucosa and enhanced the image contrast between any inflammatory lesions and the surrounding mucosa (Figure 6a- 6c, Video 4]. Consistently, the endoscopic diagnosis of BARGHett's esophagus Prague C0M1 was made and multiple targeted biopsies were obtained from any columnar appearing mucosa [16]. The subsequent histopathological analysis confirmed the presence of intestinal metaplasia with no dysplastic change.


Given the history of Helicobacter pylori-associated atrophic gastritis, attention was payed during the gastric inspection of this patient. Nonetheless, HD white-light endoscopic imaging with or without i-scan digital chromoendoscopy revealed mild hyperemia in the antrum with no macroscopic sign of atrophy or metaplasia. When turning on the OE mode 1, visible vessels appeared upon the gastric mucosa together with small flat lesions characterized by a patchy distribution and by a subtle columnar appearing morphology consistent with the macroscopic appearance of atrophic gastritis with intestinal metaplasia [Figure 7a & 7b, Video 5] [17]. Driven by these findings, targeted and four non-targeted biopsies of two topographic sites [at the lesser and greater curvature, from both the antrum and the corpus) were taken and labelled in separate vials according to international guidelines [15]. Notably, histopathology confirmed the presence of atrophic gastritis with intestinal metaplasia on OE-targeted biopsies only.
Discussion
In the last decade, with the introduction of innovative andpioneering endoscopic technologies [e.g., NBI, i-scan, FICE,optical magnification), has improved diagnostic and lesion assessment potential of gastrointestinalendoscopy [10,18].Despite the promising results coming from several clinical trials, the role of such advanced endoscopic imaging techniques ineveryday clinical practice is not well known and less defined. Therefore, the duty of modern endoscopists is to understand whether these ground breaking diagnostic techniques may refine the endoscopic accuracy or predict the microscopic assessment, thereby promoting early intervention and “treat-to-target” strategies. The Optical Enhancement [OE] technology is a dye-less chromoendoscopy techniques that engages digital post-processing to enhance mucosal contrast to better visualize gastro intestinal wall aberrancies. The peculiar wavelength-controlled spectral emission of light allows OE to overcome certain limitations associated with the use of the first generation of dye-less chromoendoscopy techniques. As a result, the mucosal vascularization acquires a sharper appearance as compared to standard white-light imaging with digital chromoendoscopy, while maintaining highlevel of image contrast and brightness to assess the mucosal pattern [12]. Whether this new advanced endoscopic imaging technique may improve the real time endoscopic definition of inflammatory and a dysplastic change is under evaluation. Very recently, Iacucci et al. [14] have successfully adopted the OE technology to assess the endoscopic disease activity in 41 patients with ulcerative colitis and 9 healthy controls [14]. Most patients with a complete endoscopic remission [MayoE 0] under HD white-light imaging revealed slight mucosal and vascular abnormalities consistent with mild active inflammation on OE imaging. Accordingly, the proposed OE score system focusing on subtle mucosal and vascular changes correlated well with either the ECAP (Extent, Chronicity, Activity, Plus additional findings) or the RHI [Robarts Histopathology Index] scores of chronic and acute microscopic changes in patients with ulcerative colitis (r=0.70 [P<0.001], and r=0.65-0.71 [P<0.001], respectively).
In our consecutive case series, we evaluated for the first time the potential impact of OE technology in every-day clinical practice. An improved management was clearly observed in four cases out of twenty consecutive endoscopic procedures conducted during a very limited period by the same operator. Notably, such refinedendoscopic definition had an impact on the clinicalmanagement in three of them.
The OE technology, especially the OE mode 2, served as an optimal guide for targeted biopsy sampling by enabling the visualization of subtle mucosal abnormalities despite negative standard HD white-light imaging and i-scan inspection. The usefulness of EO-guided biopsy appeared paradigmatic for the clinical management of a patient with a previous diagnosis of MALT lymphoma. Following Helicobacter Pylori eradication and mucosal healing, the OE targeted biopsy unveiled the presence of residual neoplastic tissue in a confined subcardial area. The targeted biopsies driven by OE technology allowed for a tailored and early treatment strategy, while the multiple random biopsies performed according to current guidelines failed to rule out the risk of endoscopically invisible residual lymphoma [21]. Similarly, OE mode 1 has proven its potential driving the histopathological diagnosis for the detection and staging of BARGHett's esophagus and gastric intestinal metaplasia, thus suggesting a greater usefulness, when compared to other endoscopic advanced techniques [22-24].
Within the colon-rectum, the OE technology, and particularly the OE-mode 1, has been successfully used to characterize the mucosal vascularization, which often represent the only endoscopic feature associated with the underling presence of subtle inflammatory changes [25]. Based on the assessment of the mucosal vascular pattern, we identified a very confined intestinal manifestation of systemic vasculitis and predict the histologic healing in ulcerative colitis.
In conclusions, this initial experience from everyday clinical practice suggest that the newly introduced OE technology supports the visualization and delimitation of subtle mucosal changes referring to either inflammatory or precancerous conditions, therefore improving both the real time diagnostic definition and the accuracy of biopsy sampling. In view our result in this case series, we feel OE technology soon will earn a placeAlong with other dye-less based chromoendoscopy techniques.However, we strongly propose the need of further assessments, with largerandomized studies, to establish whether the OE technology couldreally represent a significant improvement, when compared tocurrent dye-less and dye-based chromoendoscopic techniques.
References
- Kaminski MF, Hassan C, Bisschops R, Pohl J, Pellisé M, et al. (2014) Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 46(5): 435-449.
- Tontini GE, Vecchi M, Neurath MF, Neumann H (2013) Review article: newer optical and digital chromoendoscopy techniques vs. dye-based chromoendoscopy for diagnosis and surveillance in inflammatory bowel disease. Aliment Pharmacol Ther 38(10): 11981208.
- Basford PJ, Wheaton GL, Higgins B, Bhandari P (2014) High-definition endoscopy with i-Scan for evaluation of small colon polyps: the HiSCOPE study. Gastrointest Endosc 79(1): 111-118.
- East JE, Vleugels JL, Roelandt P, Bhandari P, Bisschops R, et al. (2016) Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy 48(11): 1029-1045.
- ASGE Technology Committee, Manfredi MA, Abu Dayyeh BK, Bhat YM, Chauhan SS, et al. (2015) Electronic chromoendoscopy. Gastrointest Endosc 81(2): 249-261.
- Rath T, Tontini GE, Nagel A, Vieth M, Zopf S, et al. (2015) High-definition endoscopy with digital chromoendoscopy for histologic prediction of distal colorectal polyps. BMC Gastroenterol 15: 145.
- Patel SG, Schoenfeld P, Kim HM, Ward EK, Bansal A, et al. (2016) Realtime characterization of diminutive colorectal polyp histology using nARGHow-band imaging: implications for the resect and discard strategy. Gastroenterology 150(2): 406-418.
- Boal Carvalho P, Magalhaes J, Dias de Castro F, Gonsalves TC, Rosa B, et al. (2016) Virtual chromoendoscopy improves the diagnostic yield of small bowel capsule endoscopy in obscure gastrointestinal bleeding. Dig Liver Dis 48(2): 172-175.
- Kominami Y, Yoshida S, Tanaka S, Sanomura Y, Hirakawa T, et al. (2016) Computer-aided diagnosis of colorectal polyp histology by using a realtime image recognition system and nARGHow-band imaging magnifying colonoscopy. Gastrointest Endosc 83(3): 643-649.
- Tontini GE, Rath T, Neumann H (2016) Advanced gastrointestinal endoscopic imaging for inflammatory bowel diseases. World J Gastroenterol 22(3): 1246-1259.
- Osawa H, Yamamoto H (2014) Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc 26( Suppl 1): 105-115.
- Neumann H, Fujishiro M, Wilcox CM, Monkemuller K (2014) Present and future perspectives of virtual chromoendoscopy with i-scan and optical enhancement technology. Dig Endosc 26(Suppl 1): 43-51.
- Nagao M, Nishikawa J, Ogawa R, Sasaki S, Nakamura M, et al. (2016) Evaluation of the Diagnostic Ability of Optical Enhancement System in Early Gastric Cancer Demarcation. Gastroenterol Res Pract 2016(2016): 1-6.
- Iacucci M, Kiesslich R, Gui X, Panaccione R, Heatherington J, et al. (2017) Beyond white light: optical enhancement in conjunction with magnification colonoscopy for the assessment of mucosal healing in ulcerative colitis. Endoscopy 49(6): 553-559.
- Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro- Soares M, et al. (2012) Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 44(1): 74-94.
- Ghaus S, Neumann H, Muhammad H, Tontini GE, Ishaq S (2016) Diagnosis and surveillance of bARGHett's esophagus: addressing the transatlantic divide. Dig Dis Sci 61(8): 2185-2193.
- Tontini GE, Lindner A, Vieth M, Vecchi M, Neurath MF, et al. (2014) Dual Focus-NARGHow Band Imaging for the detection of intestinal metaplasia and atrophic gastritis. Endoscopy 46(Suppl 1): E47-E48.
- Tontini GE, Pastorelli L, Ishaq S, Neumann H (2015) Advances in endoscopic imaging in ulcerative colitis. Expert Rev Gastroenterol Hepatol 9(11): 1393-1405.
- Yung DE, Boal Carvalho P, Giannakou A, Kopylov U, Rosa B, et al. (2017) Clinical validity of flexible spectral imaging color enhancement (FICE) in small-bowel capsule endoscopy: a systematic review and metaanalysis. Endoscopy 49(3): 258-269.
- Ishaq S, Siau K, HARGHison E, Tontini GE, Hoffman A, et al. (2017) Technological advances for improving adenoma detection rates: The changing face of colonoscopy. Dig Liver Dis 49(7): 721-727.
- Zucca E, Bergman CC, Ricardi U, Thieblemont C, Raderer M, et al. (2013) ESMO Guidelines Working Group. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6): vi144-iv148.
- Sharma P, Hawes RH, Bansal A, Gupta N, Curvers W, et al. (2013) Standard endoscopy with random biopsies versus nARGHow band imaging targeted biopsies in BARGHett's oesophagus: a prospective, international, randomised controlled trial. Gut 62(1): 15-21.
- Hoffman A, Korczynski O, Tresch A, Hansen T, Rahman F, et al. (2014)Acetic acid compared with i-scan imaging for detecting BARGHett's esophagus: a randomized, comparative trial. Gastrointest Endosc 79[1]: 46-54.
- di Pietro M, Chan D, Fitzgerald RC, Wang KK [2015] Screening for BARGHett's Esophagus. Gastroenterology 148[5]: 912-923.
- Gong EJ, Kim do H, Chun JH, Ahn JY, Choi KS, et al. (2016) Endoscopic Findings of Upper Gastrointestinal Involvement in Primary Vasculitis. Gut Liver 10[4]: 542-548.






























