Aberrant Sialic Acid Expression and Its Role In Regulating Metastasis in Colorectal Cancer
Sabah Haq1,2,3, Manpreet Sambi1,3, Bessi Qorri1,3, Nicole Mendonza1,3 and Myron R Szewczuk1*
1Department of Biomedical and Molecular Sciences, Queen's University, Canada
2Department of Pathology and Molecular Medicine, McMaster University, Canada
3Contributing first authorship
Submission: September 13, 2017; Published: September 15, 2017
*Corresponding author: Myron R Szewczuk Department of Biomedical and Molecular Sciences, Queen's University, Kingston, ON K7L 3N6, Canada, Tel: +1 613 533 2457; Fax: +1 613 533 6796; Email: szewczuk@queensu.ca
How to cite this article: Haq, S., Sambi, M., Qorri, B., Mendonza, M., Szewczuk, MR. Manpreet Sambi, Bessi Qorri, Nicole Mendonza and Myron R Szewczuk. Aberrant Sialic Acid Expression and Its Role In Regulating Metastasis in Colorectal Cancer. Adv Res Gastroentero Hepatol 2017; 7(2): 555707. DOI: 10.19080/ARGH.2017.07.555707
Abstract
Elevated cell surface sialic acid expression levels on colorectal cancer cells has been associated with increased metastatic burden. Sialic acid expression influences cancer cell-cell adhesion, cell-extracellular matrix adhesion, as well as emboli formation, apoptosis and cell growth.All of these processes are associated with the metastatic cascade, ultimately resulting in the formation of successful metastatic foci. Here we briefly summarize the steps involved in the metastatic cascade, and outline the regulatory effects of sialic acid expression on the progression of metastasis as they apply to colorectal cancer. Lastly, we discuss current models available to study these interactions as they relate to metastasis.
Keywords: Gastrointestinal cancer; Colorectal cancer; Metastasis; Sialic acid; Multicellular tumor spheroids
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer [1]. Although the advent of metastasis is only observed in 20% of patients who present with this malignancy [2], metastatic disease remains the leading cause of death in patients with CRC [3]. Aberrant patterns of glycosylation have emerged as a fundamental characteristic of cancer cells [4] and glycosylation plays an important role in cancer pathogenesis, particularly in the later stages of invasion and metastasis [5,6]. Cancer progression, invasion, metastasis and chemoresistance are highly influenced by cell surface sialoglycoproteins [6,7]. The terminal monosaccharides of N-glycans are sialic acids, and cell surface receptors and adhesion molecules like growth factor receptor, integrins, laminin, and cadherins are highly sialylated. The sialylation of these molecules is significantly altered during neoplastic transformation. Therefore, understanding the underlying mechanisms of metastasis as a whole is important, particularly when determining a treatment regimen that will prove most effective. This review will focus on the metastatic cascade as it relates to cancer as a whole, the role of sialic acids in the metastasis cascade and the models that are available to study sialic acid expression and how they can be applied to the study of colorectal cancer.
Metastatic Cascade and its Regulation
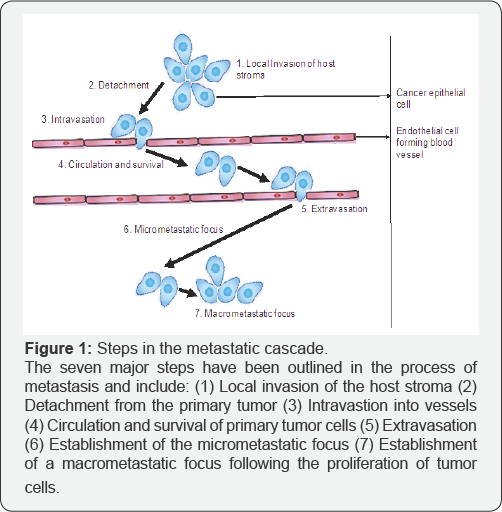
The clonal selection model of metastasis states that only a subpopulation of cells within the heterogeneous primary tumor with the metastatic genetic prerequisites are able to successfully metastasize [8,9]. Metastasis is comprised of a number of sequential steps which are detailed in Figure 1. In brief, the initial neoplastic transformation and angiogenesis results in local invasion of the host stroma by the tumor cells and is followed by detachment of these cells from the primary tumor to form tumor cell aggregates. Subsequently, intravasation of the tumor cell aggregates into the surrounding blood and lymphatic vessels allows for the detached cells to enter circulation. However, not all detached tumor cells are capable of survival in circulation, only those that are capable of surviving exit circulation from the lumina of the vessels into the parenchyma of distant tissues. These tumor cells undergo proliferation within the secondary organ parenchyma resulting in the formation of a micrometastatic foci, neovascularization and escape from the host immune response which ultimately leads to the colonization of micrometastatic foci into macroscopic tumors at the secondary site [8,10-13].
There are many mechanisms underlying each step in the metastatic cascade. Some of them include epithelial-to- mesenchymal transition (EMT), expression of matrix degrading enzymes, increased motility, evasion of apoptosis, clumping of tumor cells with platelets to form emboli, and adhesion to endothelial cell receptors [10,12,14-16]. The process of metastasis is highly dependent and regulated by interactions between cancer cells as well as between cells and the tumor microenvironment (TME) [8,10,17]. The TME is composed of a complex network of non-malignant cells, including fibroblasts, myofibroblasts, endothelial cells, mesenchymal stem cells, T-lymphocytes, B-lymphocytes, tumor associated macrophages, myeloid derived suppressor cells, adipocytes, and pericytes to name a few [8,10,17,18]. It is also composed of growth factors, cytokines and the extracellular matrix (ECM). Macrophages at the tumor periphery can stimulate invasion by secreting matrix- degrading enzymes such as metalloproteinases and cysteine cathepsin proteases [10]. As shown in Figure 1, in the last two steps the primary tumor cells must adapt to the secondary microenvironment site for successful colonization. This process is highly dependent on the metastatic cells and the secondary organ microenvironment. Paget et al. [8] eloquently describes the "seed and soil” hypothesis which explains the requisite components of the metastatic site that allow disseminated cells to establish micrometastases. In brief, the transition from epithelial (E)-Cadherin to neuronal (N)-Cadherin [19,20] and the reverse are required for cells to detach from the primary tumor and later reattach at the secondary site. This involves several processes including the recruitment of proteases such as matrix metalloproteinases (MMPs), plasmin, urokinase plasminogen activator, cathepsins and heparanases that degrade the ECM to make a path for the invading cells [21,22]. The disseminated cancer cells are then free to move forward by attaching to the ECM at the secondary site [21].
As it relates to colorectal cancer, primary tumor cells have generally been shown to metastasize to the liver [23], lung [24], peritoneum [25], brain [26] and bone [27]. In cases with single site metastases, patients can be candidates for resection of these lesions, however, for those that present with multiple metastases, determining whether these lesions can be resected successfully and improve patient survivorship is dependent on several factors including stage of CRC and toleration of the procedure [28].
Role of Sialylation in Colorectal Cancer
Glycosylation is an enzymatic process that attaches glycans to saccharides, proteins or lipids [29]. Approximately 50% of all proteins are glycosylated [30]. Adhesion proteins like integrins and E-Cadherin have N-glycans. N-linked glycans are attached to the nitrogen atom of asparagine or arginine side-chains [31,32]. Sialic acids are usually the terminal monosaccharide of N- or O-glycans. Sialic acids (neuraminic acid) are monosaccharides with a nine carbon amino sugar backbone and a carboxylic acid group at carbon-1 (C-1) position [33]. Sialic acids have three different types of α-glycosidic linkages between carbon-2 (C-2) of sialic acid and underlying sugars. The most common linkages are to the carbon-3 (C-3) or carbon-6 (C-6) positions of galactose residues giving rise to α-2,3 and α-2,6 linkages [33,34].
One of the mechanisms of sialylation in cancer cells is upregulation or altered activity of sialyltransferases via hypoxia, increased levels of androgens and the proto-oncogenes Ras and c-Myc [10,27]. Further, increased ST6Gal and subsequently synthesized levels of cell surface a-2,6-linked sialic acids have been found to be associated with metastatic spread and therapeutic failure in CRC [35]. A high concentration of sialic acids can be found in the lining of the gastrointestinal tract that are displayed on the surface of the cell, secreted as glycoproteins, and can be found in cellular secretions such as mucin [2]. However, the greatest concentration of O-acetylated sialic acids is found in the colon, with acetylation diminishing throughout developmental onset of CRC [3,4].
The precise relationship between ST6Gal and CRC can be attributed to the observed overexpression of ST6Gal in human cancers such as CRC, gastric, breast, ovarian, cervix and leukemia [35]. This in turn causes a surge in the a-2,6 sialylated N-glycan structures on the surface of cancer cells, ultimately leading to metastasis and poor prognoses. Using a mouse CRC cell line (CT26 cells), Park et al. [35] found that hepatic metastases of the CT26 cell line were entirely inhibited through the removal of the surface sialic acids. In addition, they found that the overexpression of ST6Gal in human CRC cells demonstrated critically invasive multicellular outgrowths. These findings supported their hypothesis that ST6Gal may play a role in the progression of metastasis in CRC.
The detachment of the cancer cells from the primary tumor occurs due to the reduction of cell-cell adhesion followed by an increase in cell-ECM adhesion as seen in EMT [10,36]. The homotypic and heterotypic adhesion is mediated by glycoproteins, E-Cadherins and integrins respectively. In normal cells N-glycan is essential for a5pi and E-Cadherin dimerization, cell surface expression and biological functions [30]. E-Cadherin and a5pi integrin modification by sialylation in cancer cells is instrumental in the regulation of their function [29,37,38]. However, the role of sialic acids in cancer cell-cell and cell-ECM adhesion is uncertain and a matter of controversy, with different research groups observing dissimilar results with different cancer types.
Neuraminidases are other enzymes of interest as they catalyze the hydrolytic cleavage of sialic acids from oligosaccharide chains of its respective glycoconjugates. Miyagi et al. [39] demonstrated that there are 4 types of mammalian neuraminadases, each with different roles that are involved in the progression of CRC with regard to substrate specificities and the ways in which they behave [35]. Of particular interest is Neul, a lysosomal neuraminidase that has been found to suppress CRC metastasis, whereas the plasma membrane-associated neuraminidase has been found to be upregulated in colon cancer, subsequently contributing to carcinogenesis [40,41].
An increased or aberrant expression of glycoconjugates is among the hallmarks of somatic modifications that occur in colorectal tumorigenesis. Sata et al. [42] defined two sialic acid-specific lectins, MAL and SNAI to aid in the histochemical recognition and determination of α-2,3- and α-2,6-linked sialic acids respectively. Utilization of these lectins led to the determination that healthy mucosal epithelial cells of the left and right colon were deficient in cytochemically detectable α-2,6-linked sialic acid residues, but were found to be positive for α-2,3-linked residues. However, α-2,6-linked acid after sialic residues were identified in the epithelial cells with severe dysplasia and colonic carcinomas.
Presently, the majority of studies on sialic acid expression in cell-cell and cell-ECM adhesion have been conducted on either animal models or on in vitro monolayer cell culture assays. Recently, multicellular tumor spheroids (MCTS) have emerged as a promising 3D in vitro tumor model to study cancer cell development, cell motility, metastasis and drug resistance [43,44].
Multicellular Tumor Spheroid: An Emerging Model to Study Cancer Cell Metastasis
MCTS closely resemble small avascular tumors with complex cell-cell and cell-matrix interactions [45,46], and as such is a powerful 3D in vitro model to study tumor and tumor cell proliferation, differentiation, morphology, cell invasion, metastasis, drug binding and chemoresistance [43,44,47,48]. MCTS simulate cell-cell and cell-microenvironment interactions, which are necessary for cancer cell processes like EMT, therapy resistance and metastasis. Akasov et al. [49] has developed an effective biochemical one-step highly reproducible technique of 3D MCTS formation using synthetic cyclic Arg-Gly-Asp-D-Phe-Lys (cyclo-RGDfK) sequence peptide [50]. In the biochemical method of MCTS formation, the cyclo-RGDfK binds to β1 integrin on the cell membrane, inhibiting attachment of the cells to the surface of the culture flasks and plates. The cyclo-RGDfK- α5β1 integrin interactions is followed by a delay period during which E-Cadherins are expressed on the cell membrane. Lastly, E-cadherin-E-cadherin interaction between the cells results in cell compaction and MCTS formation [49,51,52]. The cyclo- RGDfK peptide based method is a useful approach in studying the role of sialic acid in cancer metastasis.
Our group has shown that specific cell surface sialoglycan structure is correlated with the ability of breast, pancreatic and prostate cancer cells to form MCTS by the cyclo-RGDfK method. α-2,3 sialic acid promoted spheroid formation in breast and pancreatic cancer while α-2,6 sialic acid played a less significant role. Using lectin cytochemistry and flow cytometry, it was found that androgen-independent metastatic prostate cancer DU145 and PC3 cells and their drug resistant variants expressed different levels of α-2,3 and α-2,6 sialic acid residues on the cell surface which correlated with the ability to form MCTS. In prostate cancer cells, α-2,6 sialic acid residues played a greater role in spheroid formation. Our results confirmed that sialylation facilitates breast, pancreatic and prostate cancer cells to remain tightly bound in a spheroid by increasing cell-cell adhesion [53,55]. These results suggest that the relative levels of specific sialoglycan structures on the cell surface glycoproteins correlate with the ability of pancreatic, breast and prostate cancer cells to form spheroids. This area of research may also be applicable to the metastatic cascade as it relates to colorectal cancer and may provide a unique study model for the development of treatment options for this malignancy.
Conclusion
Understanding the mechanisms of metastasis is an ongoing area of research. Most cancer-related mortalities occur due to metastasis of cancer cells to distant sites. Metastatic cells are genetically unstable with various rates of proliferation, immunogenicities and diverse response to chemotherapeutics. One of the most important properties of metastatic cells is their aberrant and altered expression of surface sialylation. Indeed, sialylation plays pivotal role in many of the steps of the metastatic cascade, specifically in the progression of colorectal cancer. Exploring the different effects of sialylation on spread of colorectal cancer cells will aid in the development of alternate and novel methods of targeting cancer cell glycobiology.
Acknowledgments
This work was supported in part by grants to MR Szewczuk from the Natural Sciences and Engineering Research Council of Canada (NSERC), a private sector cancer funding from the Josefowitz Family and Encyt Technologies, Inc. to MR Szewczuk.
S Haq was the recipient of the Queen's Graduate Award (QGA, 2016) and the Ontario Graduate Scholarship (2016), and now the McMaster Medical Sciences Entrance Scholarship (2017), Research Scholarship at McMaster University (2017) and the McMaster Medical Sciences Graduate Scholarship (2017). M Sambi is a recipient of the QGA (2016). B Qorri is a recipient of the QGA (2017) and the 2017 Terry Fox Research Institute Transdisciplinary Training Program in Cancer Research. The authors report no other conflicts of interest in this work.
Authors Contribution
All authors contributed equally toward drafting and critically revising the paper and agree to be equally accountable for all aspects of the work.
References
- Gansler T, Ganz PA, Grant M, Greene FL, Johnstone P, et al. (2010) Sixty years of CA: a cancer journal for clinicians. CA Cancer J Clin 60(6): 345-350.
- van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, et al. (2015) Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis 32(5): 457-465.
- Van Cutsem E, Oliveira J, Group EGW (2009) Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO 20(Suppl 4): 61-63.
- Vajaria BN, Patel PS (2017) Glycosylation: a hallmark of cancer? Glycoconj J 34(2): 147-156.
- Stowell SR, Ju T, Cummings RD (2015) Protein glycosylation in cancer Annu Rev Pathol 10: 473-510.
- Munkley J, Mills IG, Elliott DJ (2016) The role of glycans in the development and progression of prostate cancer. Nat Rev Urol 13(6): 324-333.
- Bull C, Stoel MA, den Brok MH, Adema GJ (2014) Sialic acids sweeten a tumor's life. Cancer Res 74(12): 3199-3204.
- Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70(14): 56495669.
- Chung LW, Baseman A, Assikis V, Zhau HE (2005) Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol 173(1): 10-20.
- Hanahan D, Weinberg RA (2011) Hallmarks of Cancer: The Next Generation. Cell 144(5): 646-674.
- Yin JJ, Pollock CB, Kelly K (2005) Mechanisms of cancer metastasis to the bone. Cell Res 15(1): 57-62.
- Fidler IJ, Kripke ML (2015) The challenge of targeting metastasis. Cancer Metastasis Rev 34(4): 635-641.
- Arya M, Bott SR, Shergill IS, Ahmed HU, Williamson M, et al. (2006) The metastatic cascade in prostate cancer. Surg Oncol 15(3): 117-128.
- Bourboulia D, Stetler-Stevenson WG (2010) Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 20(3): 161-168.
- Sossey-Alaoui K, Ranalli TA, Li X, Bakin AV, Cowell JK (2005) WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res 308(1): 135-145.
- O'Shea LK, Abdulkhalek S, Allison S, Neufeld RJ, Szewczuk MR (2014) Therapeutic targeting of Neu1 sialidase with oseltamivir phosphate (Tamiflu(R)) disables cancer cell survival in human pancreatic cancer with acquired chemoresistance. OncoTargets and therapy 7: 117-134.
- Corn PG (2012) The tumor microenvironment in prostate cancer: elucidating molecular pathways for therapy development. Cancer Manag Res 4: 183-193.
- Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125(Pt 23): 5591-5596.
- Jennbacken K, Tesan T, Wang W, Gustavsson H, Damber JE, et al. (2010) N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr Relat Cancer 17(2): 469-479.
- Tomita K, van Bokhoven A, van Leenders GJ, Ruijter ET, Jansen CF, et al. (2000) Cadherin switching in human prostate cancer progression. Cancer Res 60(13): 3650-3654.
- Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12(8): 895-904.
- Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3(5): 362-374.
- Sheth KR, Clary BM (2005) Management of hepatic metastases from colorectal cancer. Clin Colon Rectal Surg 18(3): 215-223.
- Galandiuk S, Wieand HS, Moertel CG, Cha SS, Fitzgibbons RJ, et al. (1992) Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet 174(1): 27-32.
- Jayne DG, Fook S, Loi C, Seow-Choen F (2002) Peritoneal carcinomatosis from colorectal cancer. Br J Surg 89(12): 1545-1550.
- Schouten LJ, Rutten J, Huveneers HA, Twijnstra A (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94(10): 2698-2705.
- Katoh M, Unakami M, Hara M, Fukuchi S (1995) Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol 30(5): 615-618.
- Vatandoust S, Price TJ, Karapetis CS (2015) Colorectal cancer: Metastases to a single organ. World J Gastroenterol 21(41): 1176711776.
- Pinho SS, Reis CA (2015) Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 15(9): 540-555.
- Zhao Y, Sato Y, Isaji T, Fukuda T, Matsumoto A, et al. (2008) Branched N-glycans regulate the biological functions of integrins and cadherins. FEBS J 275(9): 1939-1948.
- AV Essentials of Glycobiology (1999) Cold Spring Harbour Laboratory Press, USA.
- Janik ME, Litynska A, Vereecken P (2010) Cell migration-the role of integrin glycosylation. Biochim Biophys Acta 1800(6): 545-555.
- Varki A, Schauer R (2009) Sialic Acids. In: Varki A, Cummings RD, Esko JD, et al., eds. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor (NY).
- Stencel-Baerenwald JE, Reiss K, Reiter DM, Stehle T, Dermody TS (2014) The sweet spot: defining virus-sialic acid interactions. Nat Rev Microbiol 12(11): 739-749.
- Park JJ, Lee M (2013) Increasing the alpha 2, 6 sialylation of glycoproteins may contribute to metastatic spread and therapeutic resistance in colorectal cancer. Gut Liver 7(6): 629-641.
- Creighton CJ, Gibbons DL, Kurie JM (2013) The role of epithelial-mesenchymal transition programming in invasion and metastasis: a clinical perspective. Cancer Manag Res 5: 187-195.
- Carvalho S, Catarino TA, Dias AM, Kato M, Almeida A, et al. (2016) Preventing E-cadherin aberrant N-glycosylation at Asn-554 improve its critical function in gastric cancer. Oncogene 35(13): 1619-1631.
- Dennis JW, Granovsky M, WARGHen CE (1999) Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta 1473(1): 21-34.
- Miyagi T, Yamaguchi K (2012) Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology 22(7): 880-896.
- Uemura T, Shiozaki K, Yamaguchi K, Miyazaki S, Satomi S, et al. (2009) Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene 28(9): 1218-1229.
- Kakugawa Y, Wada T, Yamaguchi K, Yamanami H, Ouchi K, et al. (2002) Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc Natl Acad Sci USA 99(16): 10718-10723.
- Sata T, Roth J, Zuber C, Stamm B, Heitz PU (1991) Expression of alpha 2,6-linked sialic acid residues in neoplastic but not in normal human colonic mucosa. A lectin-gold cytochemical study with Sambucus nigra and Maackia amurensis lectins. Am J Pathol 139(6): 1435-1448.
- Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, et al. (2010) Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol 148(1): 3-15.
- Haycock JW (2011) 3D cell culture: a review of current approaches and techniques. Methods Mol Biol 695: 1-15.
- Carver K, Ming X, Juliano RL (2014) Multicellular tumor spheroids as a model for assessing delivery of oligonucleotides in three dimensions. Mol Ther Nucleic Acids 3: e153.
- Horning JL, Sahoo SK, Vijayaraghavalu S, Dimitrijevic S, Vasir JK, et al. (2008) 3-D tumor model for in vitro evaluation of anticancer drugs. Mol Pharm 5(5): 849-862.
- Raghavan S, Mehta P, Horst EN, Ward MR, Rowley KR, et al. (2016) Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity. Oncotarget 7(13): 16948-16961.
- Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA (2009) Spheroid- based drug screen: considerations and practical approach. Nat Protoc 4(3): 309-324.
- Lin RZ, Chang HY (2008) Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J 3(9-10): 1172-1184.
- Akasov R, Zaytseva-Zotova D, Burov S, Leko M, Dontenwill M, et al. (2016) Formation of multicellular tumor spheroids induced by cyclic RGD-peptides and use for anticancer drug testing in vitro. Int J Pharm 506(1-2): 148-157.
- Lin RZ, Chou LF, Chien CC, Chang HY (2006) Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and beta1-integrin. Cell Tissue Res 324(3): 411-422.
- Luebke-Wheeler JL, Nedredal G, Yee L, Amiot BP, Nyberg SL (2009) E-cadherin protects primary hepatocyte spheroids from cell death by a caspase-independent mechanism. Cell Transplant 18(12): 1281-1287.
- Akasov R, Haq S, Haxho F, Samuel V, Burov SV, et al. (2016) Sialylation transmogrifies human breast and pancreatic cancer cells into 3D multicellular tumor spheroids using cyclic RGD-peptide induced selfassembly. Oncotarget 7(40): 66119-66134.
- Sambi M, Haq S, Samuel V, Qorri B, Haxho F, et al. (2017) Alternative therapies for metastatic breast cancer: multimodal approach targeting tumor cell heterogeneity. Breast cancer 9: 85-93.
- Haq S, Samuel V, Haxho F, Akasov R, Leko M, et al. (2017) Sialylation facilitates self-assembly of 3D multicellular prostaspheres by using cyclo-RGDfK(TPP) peptide. Onco Targets Ther 10: 2427-2447.






























